Introduction
Screening for new candidate drugs in binding assays is a standard procedure in the pharmaceutical industry. However, there are increasing requirements on assays and instrumentation. Especially the number of samples measured per day should be further increased. This can be realized if high condensed microplates are used, e.g. 1536-well microplates. The instrument of choice must be able to measure such microplates with the fastest possible read time while the assay performance criteria (such as Z´value or S/B) shall be reached.
To meet the requirements for ultra high-throughput screening BMG LABTECH has developed the microplate reader PHERAstar FSX. The new TRF laser enables fastest read times in 1536-well microplates. Two HTRF assays from Cisbio Bioassays were selected to highlight the enhanced performances achieved with the PHERAstar FSX.
Assay Principle
The HTRF® IP-One terbium cellular assay is the assay of choice to detect levels of myo-Inositol 1 phosphate (IP1) directly in cultured cells. Cells will produce IP1 following a GPCR ligand binding event on a Gq coupled receptor. This assay can be used to study the effect of GPCR agonists and antagonists on the Gq pathway. The assay principle is shown in Fig. 1.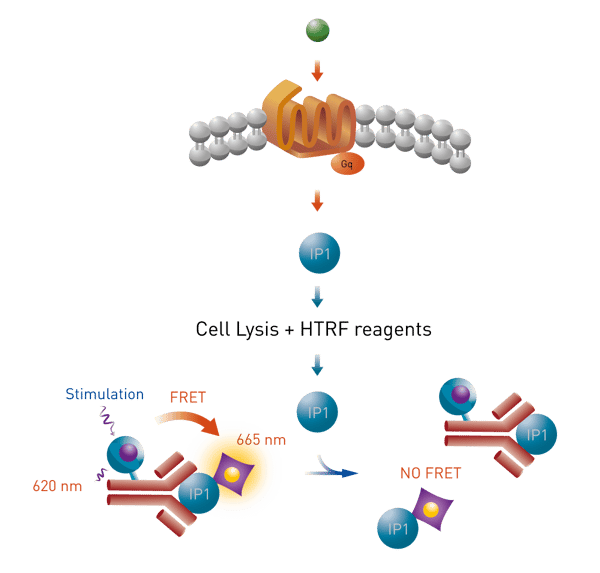
A monoclonal antibody that is specific for IP1 is labeled with the HTRF donor (Lumi4™-Tb cryptate). Intracellular produced IP1 will compete with HTRF acceptor-labeled IP1 for the antibody binding sites. The specific HTRF signal is inversely proportional to the concentration of cellular IP1 in the sample.
The second assay detects the interaction of a protein binding domain with a peptide. The assay principle is shown in Fig. 2.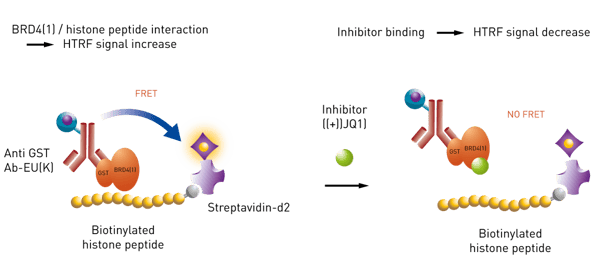 An anti GST antibody carrying the HTRF donor will bind to a GST tagged bromodomain module (BRD4). A biotinylated histone peptide is bound to the HTRF acceptor. As long as there is interaction between histone peptide and bromodomain a high HTRF signal will be monitored. Inhibitors, such as (+) JQ1, will prevent the interaction leading to a decrease in HTRF signal.
An anti GST antibody carrying the HTRF donor will bind to a GST tagged bromodomain module (BRD4). A biotinylated histone peptide is bound to the HTRF acceptor. As long as there is interaction between histone peptide and bromodomain a high HTRF signal will be monitored. Inhibitors, such as (+) JQ1, will prevent the interaction leading to a decrease in HTRF signal.
Materials & Methods
- White 1536-well Hi-Base microplates from Greiner
- HTRF IP-One assay and EPIgeneousTM Histone/BRD4 Binding assay from Cisbio
- Certus nano liquid dispenser from Gyger
- PHERAstar FSX microplate reader from BMG LABTECH equipped with flash lamp and TRF laser
|
Detection Method: |
Time resolved fluorescence (dual emission) |
|
Optic module: |
HTRF |
|
Integration start: |
60 μs |
|
Integration time: |
400 μs |
|
Light source: |
Flash Lamp or Laser |
|
Ratio multiplier: |
10000 |
Results & Discussion
IP-One cellular assay
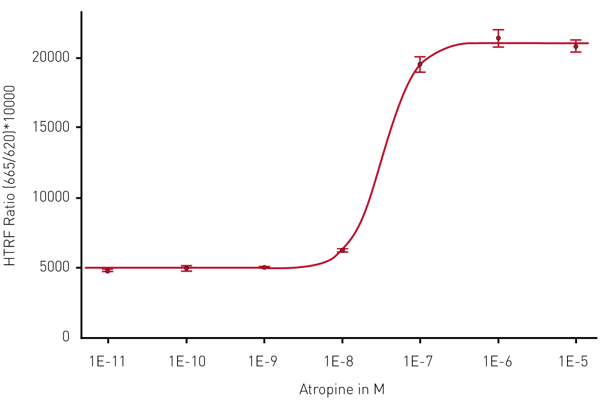
Figure 3 shows the concentration dependent effect of atropine on the level of IP-One.
The measurement was done using the TRF laser in 1 flash flying mode. The 4-parameter standard curve fit shows an R2 value of 0.9994. From the fit the MARS Data Analysis software calculated the IC50 value to be 33 nM which meets our expectations.
Histone-BRD4-Interaction assay
The known inhibitor (+) JQ1 was used to show the inhibiting effect on the histone-BRD4 interaction (Fig. 4).
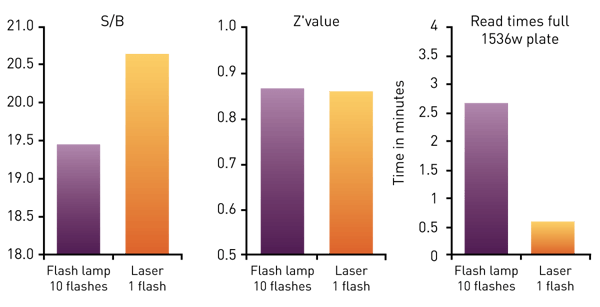
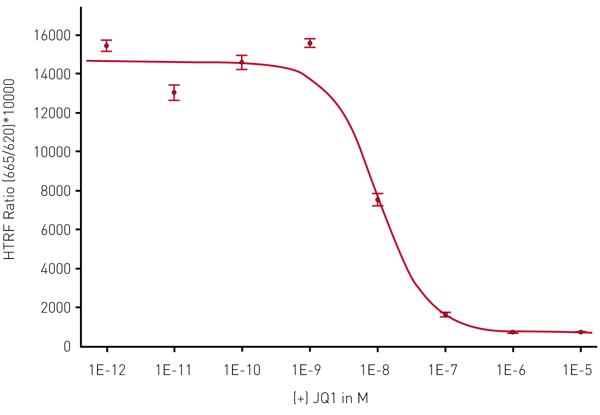 The above figures show that the HTRF assays can be reliably measured in 1536-well format with the PHERAstar FSX. Using the TRF laser in flying mode for the HTRF measurement will enable the user to read the plate in only 36 seconds while Z´value is excellent. From the data we can also follow that using the laser will lead to higher S/B values compared with flash lamp measurements (Fig. 5).
The above figures show that the HTRF assays can be reliably measured in 1536-well format with the PHERAstar FSX. Using the TRF laser in flying mode for the HTRF measurement will enable the user to read the plate in only 36 seconds while Z´value is excellent. From the data we can also follow that using the laser will lead to higher S/B values compared with flash lamp measurements (Fig. 5).
Conclusion
The combination of the HTRF technology from Cisbio with the PHERAstar FSX microplate reader provides reliable tools to fast and reliable high-throughput screening and compound follow up.
Lumi4 is a trademark of Lumiphore Inc.
HTRF is a registered trademark of Cisbio.