Introduction
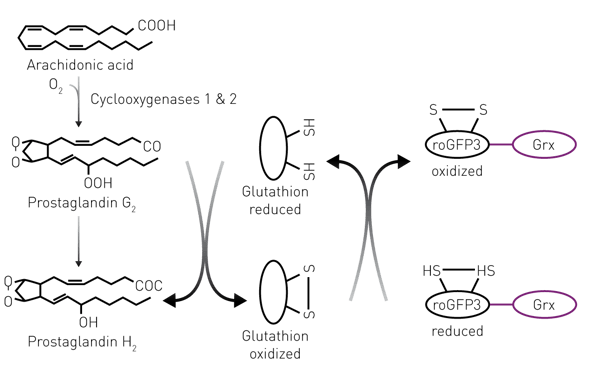
Residues of human pharmaceuticals, such as NSAIDs and their metabolites, are increasingly found in effluents of municipal wastewater treatment plants (WWTP) all over Europe. NSAIDs reduce pain and inflammation by inhibiting cyclooxygenases (COX) and thereby the production of prostanoids such as prostaglandins. The amino acid sequence of COX targeted by NSAIDs is evolutionarily highly conserved among vertebrates. Thus, it appears likely that organisms expressing COX, also physiologically respond to NSAIDs when exposed to them. For risk assessment one has to know the extent to which aquatic organisms are exposed to NSAIDs as well as metabolites with the same mode of action. Therefore, we developed and validated a cell-based mode-of-action (MOA) assay, by which the total NSAID activity in a wastewater sample enriched by solid phase extraction (SPE) can be measured as equivalents of the lead substance diclofenac (DicEQ).
Assay Principle
Materials & Methods
- CHO cells (DSMZ-No. ACC-110)
- Microplate (96-well, black bottom, GREINER)
- Diclofenac (Cayman Chemical Company)
- Arachidonic acid (Sigma)
- Plasmids (SIZ Zellkulturtechnik Mannheim)
- CLARIOstar microplate reader (BMG LABTECH)
Experimental Procedure
CHO cells were cotransfected with the genetically encoded fluorescent redox sensor Glutaredoxin-(Grx-) roGFP3 and COX1 using FUGENE-HD (Promega). Upon selection with G418 and hygromycin, cells were expanded, subcloned and screened for COX1 activity1. The COX1-dependent oxidation of the redox sensor Grx-roGFP3 increases the excitation fluorescence ratio 395nm/ 485nm, while NSAIDs concentration-dependently limit the increase. The Grx-roGFP3-COX1 sensor cell line was used to assay for NSAID activities in wastewater samples. Measurements were done with the CLARIOstar plate reader using the settings indicated below. Activities were measured in diclofenac equivalents (DicEQ).
Instrument settings
|
Optic settings |
Fluorescence intensity, well scan |
|
|
topic |
||
|
No. of multichromatic |
2 |
|
|
Monochromator Settings |
Oxidized roGFP |
|
|
Recuced roGFP |
||
|
Focal height |
4.7 mm |
|
|
Well scan |
Orbital averaging |
Orbital averaging |
|
Kinetic setting |
No. of flashes |
20 |
| Number of intervals |
3 (kinetic window 1) |
|
|
Interval time |
5 s |
|
|
Injection
|
Injection time |
After 4th cycle |
|
Injected volume |
40 µL |
|
|
Pump speed |
430 µl/s |
|
|
Shake after injection |
1 s at 300 rpm, double orbital |
|
Results & Discussion
Exposing roGFP3- and COX1-expressing cells to WWTP effluents decreases the roGFP ratio as a result of COX1 inhibition (Fig. 3).
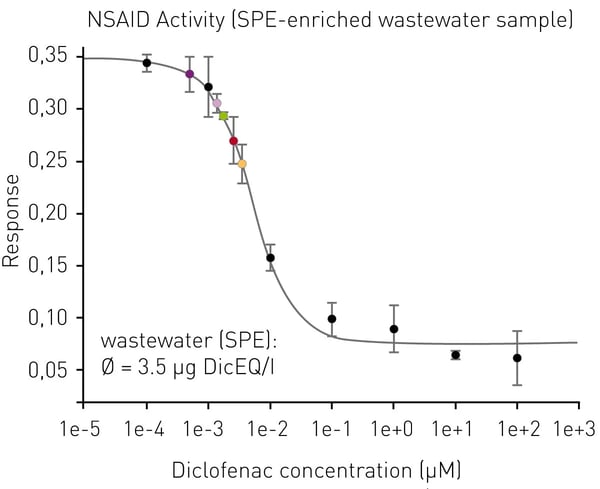
The assay was validated by the lead substance standard diclofenac of known quantity. The NSAID activity of the assayed wastewater sample was found to be 3.5 µg/l DicEQ (Fig. 4). Corresponding diclofenac concentration determined by chemical analysis (LC-MS/MS) was 2.2 µg/l (TZW Karlsruhe, data not shown). The higher NSAID activity measured by the in vitro assay of SPE-enriched wastewater compared to the diclofenac concentration determined by chemical analysis is explained by the presence of further NSAIDs. A chemical analysis of selected pharmaceutical compounds determined additional NSAIDs like ibuprofen and naproxen in the wastewater sample (TZW Karlsruhe, data not shown).
Conclusion
The MOA-based NSAID assay in living cells allows for measuring total NSAID activities in complex mixtures such as WWTP effluents as equivalents of the lead substance diclofenac (DicEQ). Further NSAIDs present in the WWTP effluent inhibited COX1 as confirmed by chemical analysis. The assay can be devised as a standard method with the potential for routine employment in large-scale monitoring of WWTP effluents and the aquatic environment they are discharged into. The CLARIOstar multi-mode microplate reader reliably detected the ratiometric roGFP probe using its unique LVF monochromatorTM. Its flexibility to record fluorescence spectra supported development of the NSAID assay.
References
- Bernhard et al. (2017) Water Research Vol. 115, pp. 74-83, May 15, 2017. doi: 10.1016/j.watres.2017.02.036