Introduction
Mycoplasma is a genus of small bacteria that lack a cell wall around their membrane1. They are well characterized as a contaminant of laboratory cell cultures. Their small size makes them difficult to detect and their lack of cell wall makes them resistant to many common antibiotics.
Mycoplasma contamination can cause serious harm to cell cultures. They have been shown to induce a variety of cellular changes and in severe cases can destroy a cell line2. The MycoAlertTM Assay is a quick and relatively easy way to detect Mycoplasma contamination. Here we show the utility of the CLARIOstar microplate reader to perform sensitive detection of this assay.
Assay Principle
The MycoAlertTM assay is a biochemical assay that selectively exploits mycoplasmal enzyme activity. Mycoplasma contamination is measured by a bioluminescent reaction that takes place only in viable mycoplasma (Fig. 1).

Measurements of luminescence are taken before and after the addition of MycoAlertTM assay substrate. Light production is quantified with a suitable luminometer, such as the CLARIOstar, to observe the presence of light emission a sample may have. Mycoplasma positive samples will produce a high luminescence signal after substrate addition resulting in a ratio of greater than 1 (ratio = reading after substrate addition/ reading before addition).
Materials & Methods
- CLARIOstar from BMG LABTECH
- Lonza MycoAlertTM Mycoplasma Detection Kit (LT07- 118)
- Lonza MycoAlertTM Assay Control Set (LT07-518) HeLa and MEF cell lines
- Greiner 96 well, F-bottom, white, microplates
MycoAlertTM assay was performed according to kit and assay control set instructions with the exception that 50 μl of samples and controls were employed.
CLARIOstar Instrument settings
| Measurement type: | Luminescence |
| Measurement mode: | Plate Mode |
| No. of cycles: | 15 (pause between 1 & 2)* |
| Cycle time: | 60 s |
| Measurement interval time: | 1.00 s |
| Focal height: | 11.0 mm |
| Gain: |
3500 |
* The assay manufacturer demands that the MycoAlertTM reagent is added manually. This action can be put into the protocol by defi ning a pause between cycle 1 and cycle 2.
Results & Discussion
As expected, addition of the substrate after the first cycle resulted in a clear increase in luminescence for positive control samples compared to the negative control (Figure 2).
The MycoAlert ratio was calculated in MARS by dividing the luminescence detected at cycle 15 by the luminescence detected at the cycle before addition of the substrate (cycle 1). As you can see in figure 3 decreasing the amount of MycoAlert positive control leads to a decreased ratio as expected.
The MycoAlert protocol states that the negative control should produce a ratio of < 0.9 while positive results should be seen for at least a dilution of 1:8 of the positive control. Using the settings shown here the CLARIOstar readings produced an average ratio of 0.09 for the negative control while the 1:8 dilution of the positive control produced an average ratio of 5.4. In fact even a dilution of 1:16 produced a positive result; 2.5 average ratio.
Finally we used MARS to calculate the MycoAlert Ratio for samples from a variety of cell lines. We used the Validations capability to give a good or bad label depending on whether the ratio was below or above 1. Figure 4 shows how this data can be depicted within the MARS software.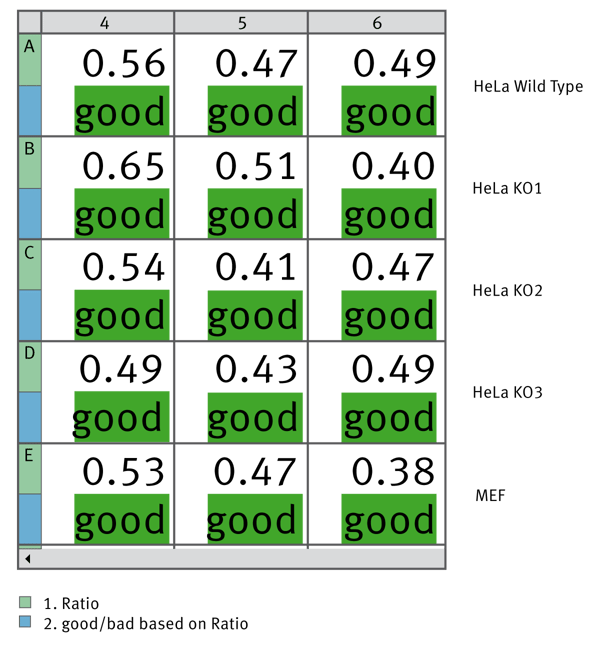
Triplicate samples from the indicated cell lines were tested for Mycoplasma contamination. Using MARS data analysis software the MycoAlert Ratio was calculated and could validate if the ratio is less than 1. All tested samples are free from Mycoplasma contamination.
Conclusion
The combination of MycoAlert assay and luminescent detection with the CLARIOstar allows users to quickly and easily check their cell cultures for possible Mycoplasma contamination. MARS calculations and validations allow users to quickly identify samples which may be contaminated.
MycoAlert is a registered trademark of LONZA
References
- S. Razin (1978). The mycoplasmas. Microbiol. Rev. 42: 414-470
- 2. I.A. Sokolova et al. (1998). Mycoplasma infection can sensitize host cells to apoptosis through contribution of apoptotic-like endonuclease(s). Immunol. Cell Biol. 76: 526-534



