Introduction
Today, luminescence-based assays are available for a broad range of applications, comprising e.g., gene reporter, cell viability and protein-protein interaction assays. These assays provide high sensitivity combined with a large dynamic range, and high-throughput compatibility on multiwell plate readers 1-3. However, when establishing these assays users are often confronted with several complications. An effect, that especially affects luminescence measurements is cross-talk.
Cross-talk paraphrases the effect, that a produced signal of a certain well is interfering with signals of its adjacent wells, resulting in artificially increased signals and reduced signal-to-blank values. Here, the cross-talk effect comprises the bleeding of a neighboring well’s signal into the optics from above the well as well as signal transmission through the well wall (fig. 1).
In this AppNote we describe three different miniaturised experiments that enable users to identify and rapidly reduce cross-talk using the example of the BacTiter-Glo™ assay.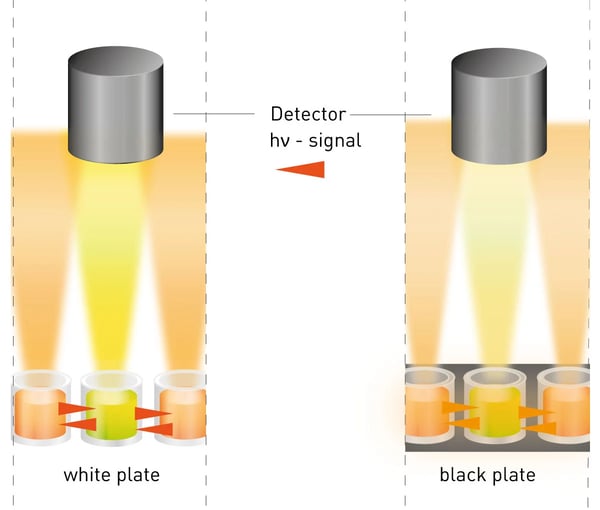
Assay Principle
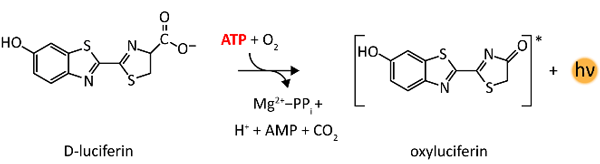
The BacTiter-Glo™ assay is a straightforward "add, mix and measure” format which couples the available amount of ATP in a sample, a parameter proportional to the number of viable cells under optimal growth conditions, to the luciferase signal using the recombinantly modified firefly Ultra-Glo™ luciferase. Here the ATP is consumed to oxidatively decarboxylate luciferin to oxyluciferin resulting in an emission of yellow-green light with the emission maximum of ~550-560 nm (fig. 2). For more details on the reaction principle see (4).
Materials & Methods
- BacTiter-Glo™ assay (#G8231, Promega)
- white/black 96 well plates (#655074/655077, Greiner)
- gray 96 well plates (#6002350, PerkinElmer)
- 100 mM ATP-stock (#R0441, Thermo Scientific™)
- CLARIOstar® Plus (BMG LABTECH)
Instrument settings
| Optic settings | Luminescence, end point | |
| Monochromator | Em 545-550 nm | |
| General settings | Measurement interval | 0.8 sec |
| Settling time | 0.3 sec | |
| Start settings | Focal height | 10 mm |
| Dynamic Range | EDR | |
| Shaking | Orbital, 10 sec, 400 rpm, before measuring | |
| Incubation | 25 °C, 5 min | |
To reduce cross-talk, users have to consider (i) the physical properties of the microplate used, influencing the signal strength, (ii) the design of the used microplate reader and (iii) the plate layout considering (i), (ii) and e.g., the expected concentrations of the target analyte.
(i) Depending on the assay mechanism different plate types are available. There are e.g., a) white plates, that enhance the produced light signal through the optical brightener TiO2; b) black plates, reducing the light intensity due to the light-absorbing effect; c) (light) gray plates, combining both properties, as they contain less or no TiO2 and are sometimes spiked with black particles (see fig.1 & 5). Additionally, wall strength and architecture of the plate are important factors when discussing cross-talk. Here, half-area well plates profit from an increased inter-well distance and lower required assay volume. In this AppNote, different plate types, layouts and the application of an aperture were evaluated regarding their support in cross-talk reduction.
Results & Discussion
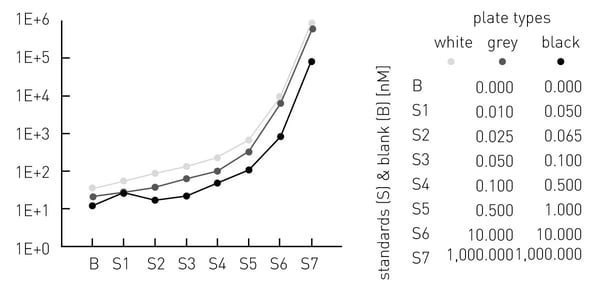
The sensitivity decreased from white via grey to black plates (fig. 3). White and grey plates offer a higher dynamic range from ~0.01-1,000 nM ATP, compared to the black plates with a dynamic range from ~0.5-1,000 nM ATP.
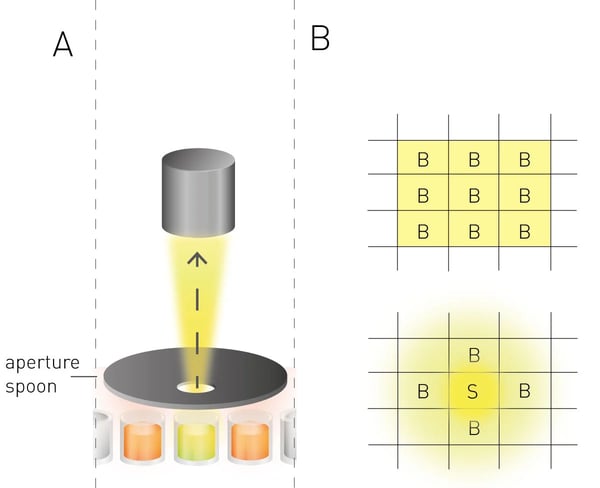
(ii) To significantly reduce the signal bleeding of adjacent wells, state-of-the-art microplate readers use apertures to physically isolate the emitted light of the target well (fig. 4A). In the case of the CLARIOstar Plus an aperture spoon is set up directly on top of the target well, letting its signal pass through a perforated screen into the optics while mostly excluding light from adjacent wells. Additionally, the CLARIOstar Plus offers a “cross-talk correction”, which mathematically corrects measured signals. Here, the signal bleeding and transmission of adjacent wells filled with blank samples were compared to blank wells situated next to wells containing a high ATP-concentration resulting in a factor dependent on the plate type (fig. 4B). Correction factors of 0.05353% for the white plates, 0.01277% for the grey plates and 0.00026% for the black plates were calculated. The increasing cross-talk effect from black via grey to white plates confirmed the results from the sensitivity measurements.
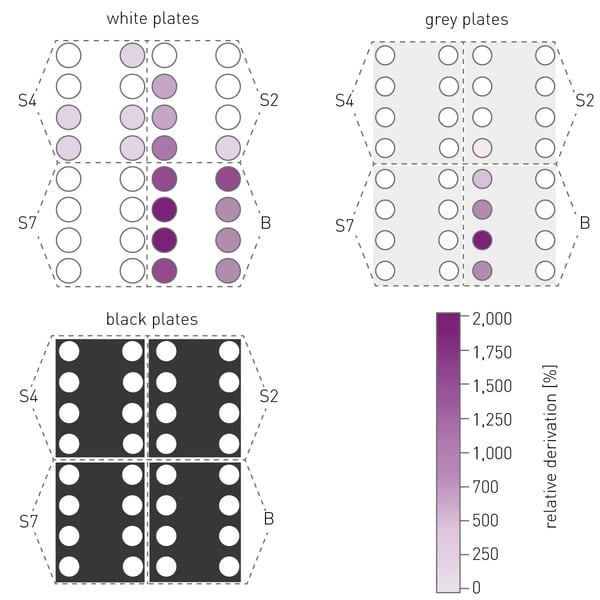
(iii) Even when considering the above-mentioned effects on cross-talk and also applying the “correction factor” as well as the aperture spoon, the plate layout still decides if a measured signal is reproducible and correct. To elucidate this effect ATP standards S2, S4, S7 and blanks (fig. 3) were plated in a certain setup (fig. 5) and their measured signals were compared to the respective signals of the respective 7-point calibration (fig. 3).
The resulting relative deviation shows that the cross-talk effect decreases from white via grey to black plates and that cross-talk might also be relevant for wells not directly adjacent to each other. In addition, large dilution steps between adjected wells should be avoided, which is evident from the strong cross-talk between S7 and blank samples.
Conclusion
The experiments shown here, highlight the influence plate type, plate layout and features of the microplate reader may have on the data quality of your luminescence results. While white plates provide the best signal output for your luminescence assays they also come with an increase in cross-talk. Appropriate plating of samples combined with the dedicated luminescence features on BMG LABTECH microplate reader can be used to minimise this problem, giving you the best possible data for your assays.
References
- Fan F, Wood KV. Bioluminescent assays for high-throughput screening. Assay Drug Dev Technol. Feb 2007;5(1):127-36. doi:10.1089/adt.2006.053.
- Remy I, Michnick SW. A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods. Dec 2006;3(12):977-9. doi:10.1038/nmeth979.
- Inglese J, Johnson RL, Simeonov A, et al. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. Aug 2007;3(8):466-79. doi:10.1038/nchembio.2007.17
- Wünsch D, et al. Luciferase-based determination of ATP/NAD(H) pools in a marine (environmental) bacterium. Microbial Physiol. 2022:1-12. doi:10.1159/000522414