Introduction
When you think about ATP, the energy-rich molecule essential for life, you are thinking about the ATP that is inside of cells. ATP outside of cells or extracellular ATP is an important signaling molecule involved in cell proliferation and other processes.1
Interest in extracellular ATP is rising due to its role in promoting the immunogenic cell death (ICD) response2. Cells that undergo ICD-driven apoptosis release extracellular ATP and other molecules known as damage associated molecular pattern (DAMP) molecules. Working together extracellular ATP and other DAMP molecules promote the innate and adaptive immune responses. It is hoped that new drugs can be discovered that stimulate extracellular ATP release by cells and subsequently drive these cells to undergo apoptosis by ICD for therapeutic purposes.
Here we present results that use Promega’s RealTime-Glo™ Extracellular ATP Assay, a simple add-mix-measure assay optimized to detect extracellular ATP from living cells in real time.
Assay Principle
Current measurements of extracellular ATP involve collection and processing of supernatant followed by end-point measurements making the process time and resource consuming1. The RealTime-Glo™ Extracellular ATP Assay is a bioluminescent assay designed for kinetic monitoring of ATP released from dying, stressed, or activated cells (Fig. 1). Extracellular ATP can be directly assessed due to a thermostable luciferase and an optimized luciferin.
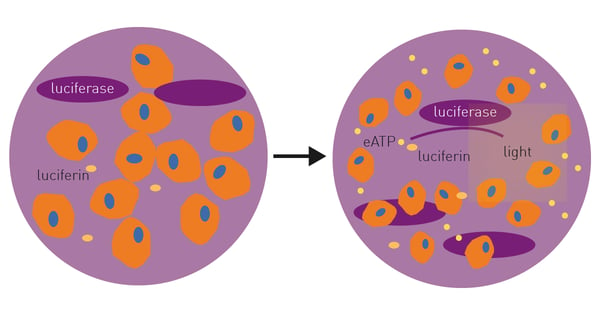 Cells are seeded into wells containing potential ICD-inducing agent and RealTime-Glo™ Extracellular ATP Assay reagents are added immediately. Changes in extracellular ATP are monitored over time in a CLARIOstar Plus microplate reader with Atmospheric Control Unit (ACU) as the effects of the ICD inducer occur.
Cells are seeded into wells containing potential ICD-inducing agent and RealTime-Glo™ Extracellular ATP Assay reagents are added immediately. Changes in extracellular ATP are monitored over time in a CLARIOstar Plus microplate reader with Atmospheric Control Unit (ACU) as the effects of the ICD inducer occur.
Materials & Methods
- Costar®, 96-well, white, clear bottom plates (Corning)
- RealTime-Glo™ Extracellular ATP Assay and RealTime- Glo™ Annexin V Apoptosis and Necrosis Assay Reagents (Promega)
- Breathe-Easy® Membranes (Diversified Biotech)
- RPMI 1640 medium +10% FBS (Gibco)
- CLARIOstar Plus with ACU (BMG LABTECH)
ICD compounds were used at the indicated concentrations and added in 50 µl volumes. U937 cells were harvested by centrifugation and resuspended in media. 50 µl volumes were used at 10,000 cells/well unless otherwise indicated. RealTime-Glo™ Extracellular ATP Assay reagent was prepared according to the manufacturer’s recommendations in media and 100 µl was dispensed. Where indicated RealTime-Glo™ Annexin V Apoptosis and Necrosis Assay Reagent was also prepared in media and added to a matching set of wells prepared with ICD compound and U937 cells. After preparation as described earlier, the microplate was sealed and moved to the CLARIOstar with ACU for measurement.
Instrument settings
|
Optic settings
|
Luminescence, plate mode kinetic
|
|
| Emission |
No filter |
|
|
Gain
|
3600 | |
|
Focal height
|
2.2 mm | |
|
General settings
|
Optic used | Bottom |
| Measurement Int. time |
1.00 s
|
|
|
Kinetic settings
|
Number of cycles |
145
|
| Cycle time |
10 min
|
|
|
Incubation
|
Target temperature |
37.0° C
|
| Target O2 |
Monitoring
|
|
| Target CO2 |
5%
|
|
Results & Discussion
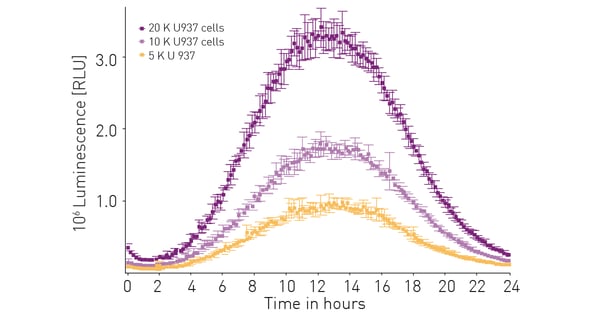
The sensitivity, stability and other performance features of the RealTime-Glo™ Extracellular ATP Assay were established previously.3 As a further validation the effect of cell number on luminescent signal was assessed. Figure 2 shows that the amount of extracellular ATP released and detected is proportional to the number of cells present.
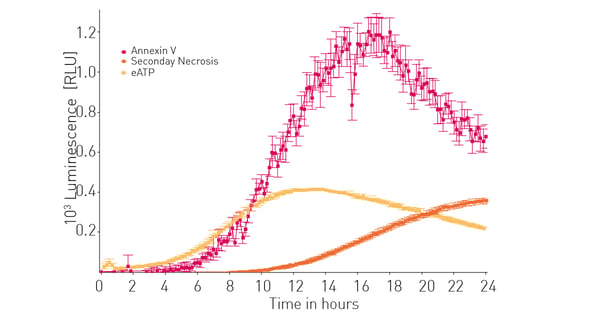
 We then compared extracellular ATP release to other known markers of apoptosis and subsequently necrosis. The CLARIOstar with ACU was previously shown to assist in detection of the RealTime-Glo™ Annexin V Apoptosis and Necrosis Assay.4 Figure 3 shows that extracellular ATP release correlates well with early apoptotic events and as expected precedes subsequent necrotic events induced by bortezimib.
We then compared extracellular ATP release to other known markers of apoptosis and subsequently necrosis. The CLARIOstar with ACU was previously shown to assist in detection of the RealTime-Glo™ Annexin V Apoptosis and Necrosis Assay.4 Figure 3 shows that extracellular ATP release correlates well with early apoptotic events and as expected precedes subsequent necrotic events induced by bortezimib.
 Next a comparison of several possible ICD inducers was made to better understand the nature of the extracellular ATP release response. Figure 4 shows that the compounds selected exhibit a variety of responses that differ in the timing of the response onset, amount of extracellular ATP released and duration of sustained release. The breadth of these results from a single experiment is a distinct benefit of the RealTime-GloTM Extracellular ATP Assay.
Next a comparison of several possible ICD inducers was made to better understand the nature of the extracellular ATP release response. Figure 4 shows that the compounds selected exhibit a variety of responses that differ in the timing of the response onset, amount of extracellular ATP released and duration of sustained release. The breadth of these results from a single experiment is a distinct benefit of the RealTime-GloTM Extracellular ATP Assay.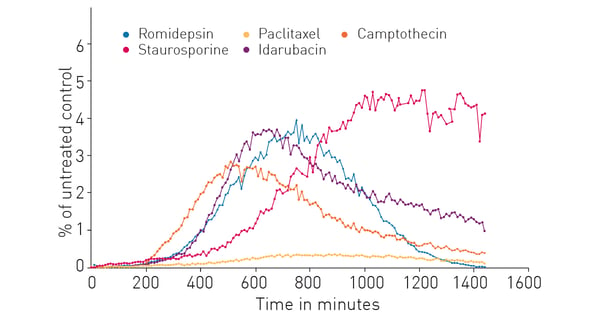
Conclusion
We show that the RealTime-GloTM Extracellular ATP Assay is a valuable tool to investigate extracellular ATP and its role in ICD. This simple add-mix-read real-time cell-based assay enables continuous measurement from a single plate, significantly reducing lab consumables. Using the CLARIOstar with ACU ensures that cells remain viable to detect changes due to drug treatment.
References
- Seminario-Vidal L., et al. (2009) Assessment of extracellular ATP Concentrations Methods Mol. Biol. -Biochemisty. 574: 25-36.
- Garg, A.D., et al. (2014) Danger signalling during cancer cell death: origins plasticity and regulation Cell Death Differ. 21: 26-38.
- Niles A.L., et al. (2021) A live-cell assay for the real-time assessment of extracellular ATP level Anal. Biochem. 628: doi: 10.1016/j.ab.2021.114286.
- Kupcho, K., et al. (2017) Real-time assessment of apoptosis and necrosis BMG LABTECH AN 311.
