Introduction
Androgen-disrupting chemicals are molecules found in the environment that interfere with male fertility by disrupting hormone signaling.1 Current screening assays for agonists and antagonists of androgen receptors are limited to time-consuming and resource-intensive in vitro tests and in vivo animal assays. Viable alternatives are therefore needed that offer high throughput capabilities for screening of androgen-disrupting chemicals and their metabolites (xenobiotics).
One type of assay that is amenable to scale and predictive value for the identification of androgen disruptors is the androgen receptor dimerization assay. Androgen receptor dimers control the expression of key genes involved in hormonal signaling and the different events linked to normal male development. Androgen receptor dimerization assays can be used to study agonists that activate the dimeric androgen receptor or antagonists which block the normal function of the active receptor.
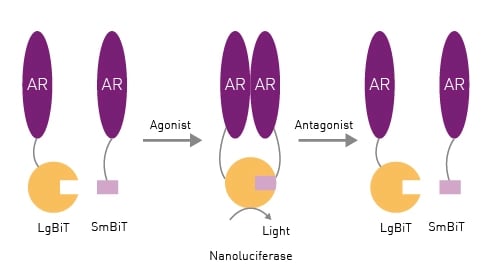
In this study, a novel cell-based receptor dimerization assay (AR2) was developed that measures ligand-dependent androgen receptor homodimerization using the NanoLuc® Binary Technology (NanoBiT®).2 When the Large BiT and Small BiT components come together, they form a functional NanoLuc luciferase enzyme suitable for determination of protein-protein interactions for the homodimer receptor (Fig.1). This system can be used to study the effects of different agonists and antagonists.
 For antagonists, fluorescent cell viability measurements were performed in parallel. These measurements permit assessment of any loss of luminescence due to antagonists impacting cell viability as opposed to androgen receptor activity. In addition, the AR2 receptor dimerization method was extended to include measurements related to cytochrome P450 (CYP) enzymes and to determine the impact of xenobiotic compounds on receptor activity.
For antagonists, fluorescent cell viability measurements were performed in parallel. These measurements permit assessment of any loss of luminescence due to antagonists impacting cell viability as opposed to androgen receptor activity. In addition, the AR2 receptor dimerization method was extended to include measurements related to cytochrome P450 (CYP) enzymes and to determine the impact of xenobiotic compounds on receptor activity.
- Solid white 384-well microplates, Greiner Bio-One
- OptiMEM medium, Life Technologies
- Human hepatocellular carcinoma cells (HepG2), American Type Culture Collection
- LgBiT and SmBiT (NanoBiT), Promega
- Furimazine, AOBIOUS
- Reazurin sodium, Thermo Fisher
- CLARIOstar Plus microplate reader, BMG LABTECH
Experimental procedure
AR2 receptor dimerization assays were performed to measure responses to different agonists and antagonists. Assessment of cell viability was added to antagonist mode tests. The complete experimental details have been previously reported.2
Instrument settings for cell viability assays
|
Optic settings
|
Fluorescence, top optic
|
|
|
Monochromator
settings |
Excitation: 530 |
|
|
Gain
|
860 |
|
|
Focal height
|
7.8 mm |
|
|
General settings
|
Number of flashes | 35 |
| Cycle time | 20 sec | |
Instrument settings for AR2 receptor dimerization assays
|
Optic settings
|
Luminescence, endpoint
|
|
|
Gain
|
||
|
General settings
|
Integration time | 0.08 sec |
Results & Discussion
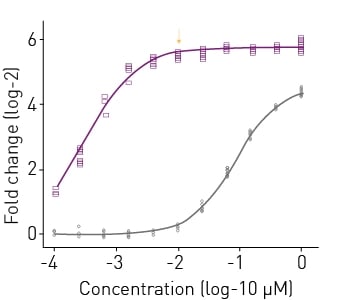
In this study, more than 100 compounds were tested in a novel androgen receptor dimerization assay for activity as agonist or antagonists. An example of agonist mode testing with AR2 receptor dimerization assays is shown in Fig.2. R1881 (metribolone) elicited a >50-fold response at concentrations above 10 nM and a half-maximal activity concentration (AC50) of 319 pM. Testosterone was not as effective as R1881, evoking only a 23-fold response. In subsequent experiments, R1881 was selected as the positive control for agonist mode experiments where test chemicals were evaluated for their ability to promote AR
receptor dimerization.
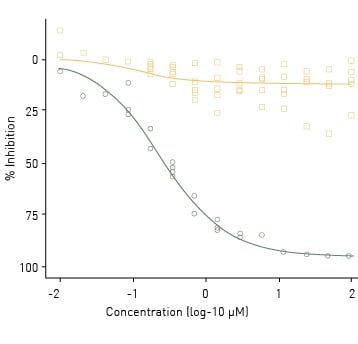
Figure 3 shows an example of antagonist mode testing using AR2 receptor dimerization assays. R1881 was co-administered with a titrated series of five known AR antagonists and a negative control for 18 h. Cyproterone acetate was the most potent AR antagonist with an AC50 of 240 nM. Amitrole was used as a negative control and did not significantly inhibit AR dimerization. Collectively the data show that the AR2 receptor dimerization assay responds predictably to both agonists and antagonists.
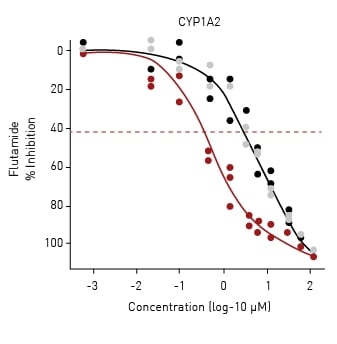
The 128 chemicals used in the study were re-tested in the presence of individual human CYP enzymes using an mRNA transfection method.3 This reevaluation changed the activity calls for five compounds (as an example, the dose-response curve for flutamide is shown in Fig.4) and shifted the estimated potencies for several others. The AR2 receptor dimerization assay was thus capable of being retrofitted to look at androgen-disrupting metabolites generated by human cytochrome P450 (CYP) enzymes.
Conclusion
The AR2 receptor dimerization assay is a robust, fast, and reliable way to screen for agonists, antagonists and xenobiotic metabolites. With robust Z’ factors of 0.94 and 0.85 for agonists and antagonists, respectively, this assay is well suited to high-throughput screening. The AR2 receptor dimerization assay successfully classified known agonists and antagonists as well as or better than legacy assays with equal or higher estimated potencies. The BMG LABTECH CLARIOstar Plus microplate reader was the ideal platform to measure luminescence changes for the NanoBiT in these cell-based assays which was crucial to the measurement of androgen disruptor activity.
References
-
Luccio-Camelo DC and Prins GS. Disruption of androgen receptor signaling in males by environmental chemicals. Journal of Steroid Biochemistry and Molecular Biology (2011) 127(1-2):74-82. doi: 10.1016/j.jsbmb.2011.04.004.
-
Brown EC et al., High-throughput AR dimerization assay identifies androgen disrupting chemicals and metabolites. Frontiers in Toxicology (2023) 5:1134783.doi:10.3389/ftox.2023.1134783.
-
DeGroot DE et al., mRNA transfection retrofi ts cellbased assays with xenobiotic metabolism. Journal of Pharmacological and Toxicological Methods (2018) 92:77-94. doi: 10.1016/j.vascn.2018.03.002.