Introduction
Ion channels are important drug targets because of their critical role in nerve, cardiac, endocrine and skeletal muscle tissues. The lack of sufficiently sensitive screening systems has hampered research in this area. This application note focuses on Voltage Sensor Probes (VSP), a Fluorescence Resonance Energy Transfer (FRET)-based voltage-sensing assay technology from Invitrogen for measuring changes in cellular membrane electrical potential.
This technology enables detection and measurement of rapid changes in membrane voltage and quickly reports them as fluorescence signals from living cells. The ratiometric method used to detect and quantify changes in cellular membrane potential significantly reduces errors arising from well-to-well variations in cell number, dye loading, and signal intensities, plate inconsistencies, and temperature fluctuations. These combined features make VSP technology highly amenable for high-throughput screening (HTS) applications.
This application note demonstrates the use of BMG LABTECH microplate readers for development of VSP ion channel assays.
Assay Principle
VSP is a Fluorescence Resonance Energy Transfer (FRET)- based assay technology used for ion channel drug discovery. The FRET donor is a membrane-bound, coumarin-phospholipid (CC2-DMPE), which binds only to the exterior of the cell membrane. The FRET acceptor is a mobile, negatively charged, hydrophobic oxonol [either DiSBAC2(3) or DiSBAC4(3)], which will bind to either side of the plasma membrane in response to changes in membrane potential. Resting cells have a relatively negative potential, so the two probes associate with the exterior of the cell membrane, resulting in efficient FRET (Figure 1). Exciting the CC2-DMPE donor probe (filter: 400-10 nm) generates a strong red fluorescence signal (filter: 570-20 nm) from the oxonol acceptor probe.
When the membrane potential becomes more positive, the oxonol probe rapidly translocates (on a subsecond time scale) to the other face of the membrane. Thus, each oxonol probe “senses” and responds to voltage changes in the cell.
This translocation separates the FRET pair, so exciting the CC2-DMPE donor probe and generates a strong blue fluorescence signal (filter: 460-10 nm) from the CC2-DMPE probe.
 The ion channel model we have used is endogenously expressed inward rectifying potassium (Kir) channels present in rat basophilic leukemia cells. These channels are opened when high potassium is added to the cell’s medium. This causes a change in the cell’s membrane potential which can be modulated by barium chloride. In the assay, the cells are manually loaded with the dyes as described below. Barium chloride is then immediately added at various concentrations. After incubation, the plated cells are placed in the reader. The reader begins to take baseline readings at the two wavelengths, injects the high potassium buffer (VSP-2 buffer, below), and continues to take readings for a specified time.
The ion channel model we have used is endogenously expressed inward rectifying potassium (Kir) channels present in rat basophilic leukemia cells. These channels are opened when high potassium is added to the cell’s medium. This causes a change in the cell’s membrane potential which can be modulated by barium chloride. In the assay, the cells are manually loaded with the dyes as described below. Barium chloride is then immediately added at various concentrations. After incubation, the plated cells are placed in the reader. The reader begins to take baseline readings at the two wavelengths, injects the high potassium buffer (VSP-2 buffer, below), and continues to take readings for a specified time.
Materials & Methods
Cell Culture
RBL-2H3 (Rat Basophilic Leukemia, ATCC) cells were plated at 50,000 cells/well in Corning® 96-well plates 18-24 hours prior to experimental procedure.
Preparation of VSP Loading Buffers
- 5 μM CC2-DMPE Loading Buffer: Premix 10 μL of 5 mM CC2-DMPE (Invitrogen) and 10 μL of 100 mg/mL Pluronic® F-127 (Sigma). Add 10 mL of VSP Solution 1 (160 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, pH 7.4) and vortex vigorously to mix. Protect from light prior to use.
- 10 μM DiSBAC2(3) Loading Buffer: Premix 8.3 μL of 12 mM DiSBAC2(3) (Invitrogen) and 12.5 μL of 200 mM VABSC-1 (Invitrogen). Add 10 mL of VSP Solution 1 and vigorously vortex to mix.
Loading Cells
Media were replaced with 100 μL VSP Solution 1 (VSP-1). The VSP-1 was immediately replaced with 100 μL CC2- DMPE Loading Buffer and incubated at room temperature for 30 minutes, covered and protected from light. The CC2-DMPE Loading Buffer was removed after 30 minutes and the plates washed once with 100 μL VSP-1. The VSP-1 was immediately replaced with 100 μL DiSBAC2 Loading Buffer and incubated at room temperature for 30 minutes, covered and protected from light.
BaCl2 (Sigma), when appropriate, was added either immediately following addition of DiSBAC2 Loading Buffer or immediately prior to VSP-2 high K+ buffer (164.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, pH 7.4).
Results & Discussion
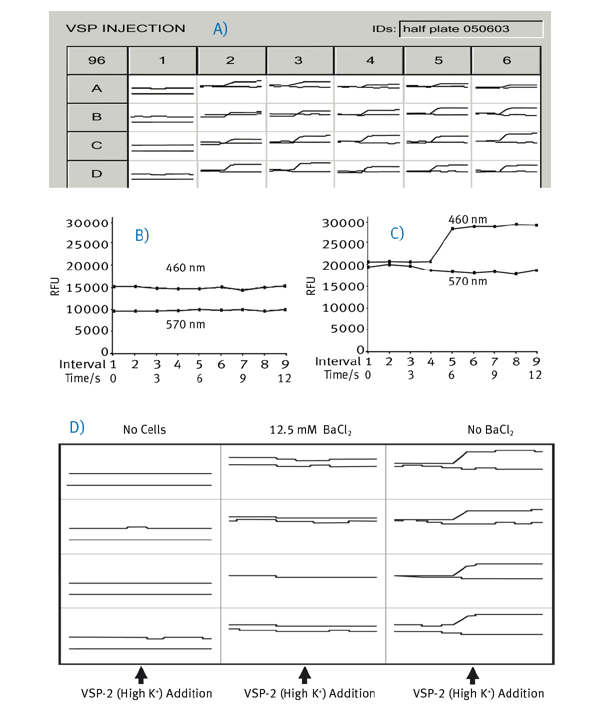
Immediately following the DiSBAC2 Loading Buffer 30 minute incubation, the plates where analyzed the BMG LABTECH microplate reader. Data are analyzed by comparing the baseline subtracted ratio of donor (at 460 nm) to acceptor (at 570 nm) before (Ro) and after (Rf) addition of VSP-2: the final normalized assay ratio = Rf/Ro. A plate readout view is shown in Figure 2A for a “positive control” assay with a 96-well plate with 50,000 RBL cells/well. Column 1 is the no cells control. Protect from light prior to use.
In Figure 2B a current state window view of a no cell control shows no change in 460 nm upon VSP-2 addition, whereas in Figure 2C an increase in the 460 nm signal upon injection of VSP-2 is detected. Figure 2D shows enlarged views of current state windows from 12 wells of a BaCl2 dose response. Note the lack of 460 nm emission increase in both the cell control and 12.5 mM BaCl2 wells compared to untreated wells.
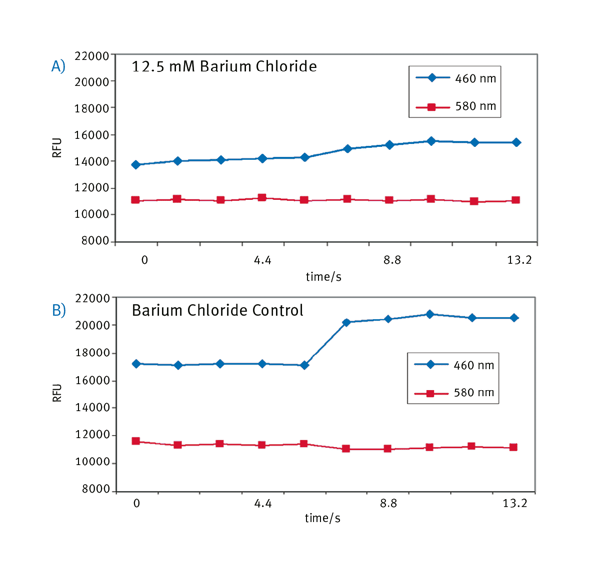
The raw fluorescent readout was evaluated in Excel and the addition of 12.5 mM BaCl2 avoids a significant change in 460 nm emission (Figure 3A), whereas an untreated control well shows as expected an increase in 460 nm fluorescence signal upon VSP-2 addition (Figure 3B).
Conclusion
This application note demonstrates the compatibility of Voltage Sensor Probe technology with plate readers from BMG LABTECH. The key to the technology is having instrumentation that can measure two wavelengths at the same time. This takes advantage of the ratiometic FRET readout, which greatly reduces variation common to many cell based assays. The ability of the BMG LABTECH readers to inject channel stimulant, switch filters at approximately 1 Hz, and continue to take readings during the kinetic event make these suitable instruments for ion channel assays.