Introduction
Protein Kinase C (PKC) enzymes are a diverse family of enzymes that under specific signaling conditions phosphorylate proteins on serine and threonine amino acids. PKCs are involved in many cellular functions including neuronal activity, cell growth, cell proliferation, and cell movement, as well as in several diseases including cardiovascular, cancer, diabetes, and Alzheimer’s. Therefore a TR-FRET screening assay, such as the one described here, can aid in further elucidating PKC’s function.
Tyrosine kinases (TKs) are a diverse family of enzymes that under specific signaling conditions phosphorylate proteins on the amino acid tyrosine. TKs are involved in many cellular functions such as cell division and cell proliferation, as well as in several diseases including cancer and diabetes. Tyrosine kinases play important roles in cell growth, thereby making them important drug targets in cancer therapy. For that reason, a TR-FRET screening assay, such as the one described here, can aid in elucidating specific inhibitors for tyrosine kinases.
Assay Principle
A fluorescein labeled poly-GT or poly-GAT (Glu, Ala, Tyr) substrate is incubated with a tyrosine kinase and ATP. For protein kinase C assays a fluorescein labeled PKC substrate is incubated with PKC and ATP. Subsequently, a terbium labeled antibody that binds to the phosphorylated form of the substrate is added. When the antibody interacts with the phosphorylated substrate, FRET will occur between the terbium label (emits at 490 nm) and the fluorescein moiety (emits at 520 nm) (Figure 1). Measurements are taken at 490 and 520 nm and the 520/490 ratio is plotted versus the concentration of enzyme to determine an EC50 for the enzyme and its substrate.
Measurements are taken at 490 and 520 nm and the 520/490 ratio is plotted versus the concentration of enzyme to determine an EC50 for the enzyme and its substrate.
Materials & Methods
- Invitrogen’s Fluorescein PKC Substrate and PKC Kinase Buffer (5x), PKCα, Kinase Quench Buffer
- Invitrogen’s LanthaScreen™ Tb-PKC Substrate Antibody
- Invitrogen’s Fluorescein-poly-GT and Fluorescein-poly-GAT, Tyrosine Kinase Buffer, ZAP70, Screen Dilution Buffer
- Invitrogen’s LanthaScreen® Tb-PY20 Antibody
- Black Corning® low volume 384-well microplate
PKC Titration
Multiple PKC isoforms were titrated to determine optimal kinase concentrations for screening. The following protocol is an example of the conditions used to determine the EC50 of PKCα and its substrate. A dilution series of PKCα, starting at a final concentration of 2.0 µg/ml, was incubated in the presence of 250 nM fluorescein-labeled PKC substrate and 20 µM ATP in a total volume of 10 µl in a microplate. After a 90-minute incubation at room temperature, 10 µl of TR-FRET dilution buffer containing 2X EDTA (20 mM) and 2X Tb-PKC antibody (1.0 nM) was added and mixed to create a final volume of 20 µl per well, a final 1X EDTA concentration of 10 mM, and a final 1X antibody concentration of 0.5 nM. After incubating for 60 minutes at room temperature, the plate was read on the BMG LABTECH microplate reader. Each data point represents the average of three wells.
Tyrosine Kinase Titration
To demonstrate the functionality of a universal tyrosine kinase assay using LanthaScreen™, both fluorescein-poly-GAT (Glu, Ala, Tyr) and fluorescein-poly-GT (Glu, Tyr) substrates were used with terbium labeled PY20 antibody.
A dilution series of kinase was incubated with 400 nM fluorescein labeled substrate and 200 µM ATP in a total volume of 10 µl in a microplate. After 60 mins of incubation at room temperature, 5 µl of TR-FRET dilution buffer containing 4X EDTA (60 mM) was added. Next, 5 µl of TR-FRET dilution buffer with 4X antibody (8 nM) was added and mixed to create a final volume of 20 µl per well, a final 1X EDTA concentration of 15 mM, and a final 1X antibody concentration of 2 nM. After 60 mins of incubation at room temperature, the plate was read in the microplate reader.
Instrument settings
|
CLARIOstar
|
PHERAstar FS
|
|
|
Detection mode
|
Time-resolved Fluorescence
|
|
|
Method
|
Endpoint, Top optic
|
|
|
Optic settings
|
Ex-Filter: Ex TR
Em-FilterA: 520-10
Em-FilterB: 490-10
|
LanthaScreen
Optic module: 337 520 490
|
|
Integration start
|
100 µs
|
|
|
Integration time
|
200 µs
|
|
Results & Discussion
Figure 2 shows representative kinase titration curves for PKCα and PKCζ.
Table 1 shows statistical data for all kinases tested.
Table 1: LanthaScreen™ on the PHERAstar: statistics for PKC isoform titrations.
| PKCα | PKCßI | PKCßII | PKCd | PKCε | PKCO | PKCζ | |
| EC50 (ng/ml) | 0.43 | 0.27 | 0.22 | 0.07 | 0.03 | 0.16 | 0.12 |
| Min. Value | 0.44 | 0.43 | 0.79 | 0.55 | 0.73 | 1.02 | 0.84 |
| Max. Value | 2.69 | 2.66 | 2.61 | 2.64 | 2.63 | 2.63 | 2.63 |
| Max-min | 2.25 | 2.23 | 1.82 | 2.09 | 1.90 | 1.61 | 1.79 |
| Fold Diff (max/min) | 6.1 | 6.2 | 3.3 | 4.8 | 3.6 | 2.6 | 3.1 |
| Hill Slope | 1.1 | 0.89 | 1.01 | 0.70 | 0.93 | 0.96 | 0.83 |
| R2 | 0.991 | 0.996 | 0.988 | 0.979 | 0.988 | 0.976 | 0.982 |
| S:N | 60 | 47 | 18 | 31 | 25 | 14 | 18 |
| Z‘-factor value | 0.94 | 0.91 | 0.78 | 0.85 | 0.85 | 0.74 | 0.79 |
All PKC isoenzymes were titrated in a similar manner as PKCα. Regardless of “fold difference” or signal-to-noise ratios, all assays provided Z’ values far greater than 0.5. This is due to the use of FRET-based, ratiometric data analysis, which leads to low standard deviations of the replicates. Both hill slope and R2 measurements fall within acceptable limits. R2 values approaching 1.0 indicate a perfect curve fit.
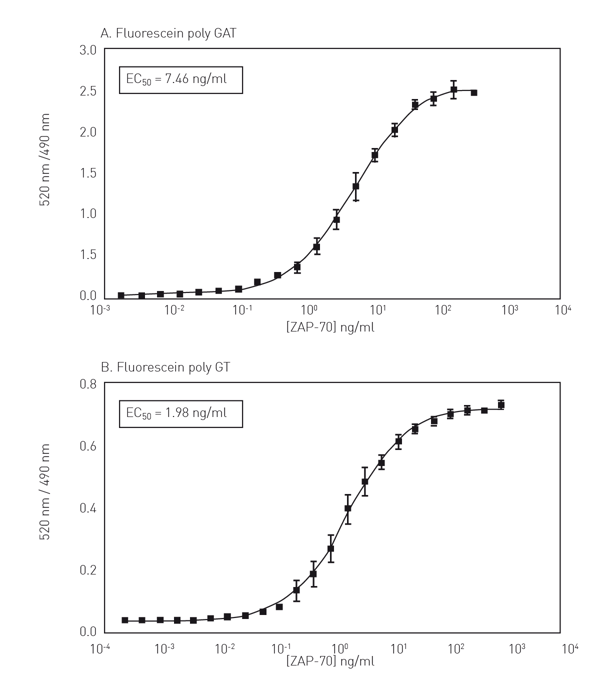
In figure 3 kinase titration curves for ZAP-70 are presented (Fig. 3A+B).
The results show that use of a fluorescein-labeled substrate common for many TKs (poly-GT or poly-GAT) allows for the development of a “universal” TK assay using the LanthaScreen™ format.
Conclusion
Invitrogen’s terbium-based LanthaScreen™ can easily be measured on BMG LABTECH instrumentation. The assay shows several advantages:
- The ratiometric nature of LanthaScreen™ assay eliminates well to well variation.
- The time-resolved nature allows for the use of fluorescein without the associated drawbacks of compound interference.
- The ability to use fluorescein simplifies assay development and costs.