Introduction
Protein kinases and their ability to phosphorylate proteins play key roles in the signal transduction pathways of many diseases such as cancer, arthritis, and diabetes. The importance of protein kinases makes them common targets for many High-Throughput Screening (HTS) departments within the pharmaceutical industry. Current screening technologies employ the use of phosphospecific antibodies or radioactive beads.
Lonza has developed PKLightTM, a non-radioactive, homogeneous, robust, and simple assay suitable for the screening of potentially all protein kinases in 96-, 384- and 1536-well formats. This technology utilises Luciferase bioluminescence to measure ATP consumption as a result of kinase phosphorylation of the target substrate. The assay can be easily optimised for each kinase/substrate pair to produce rapid, quality data suitable for IC50 determination of screen compounds.
This technology does not require antibodies, radio-active beads, radio-labelled ATP, or specifically modified substrate sequences. The signal is glow luminescence with a half life greater than 2 hours, which is detected using a luminometer, in our case the multifunctional BMG LABTECH PHERAstar FS plate reader. In this application note, we use the Ser/Thr Kinase, cAMP dependant protein kinase (PKA) to demonstrate the assay.
Assay Principle
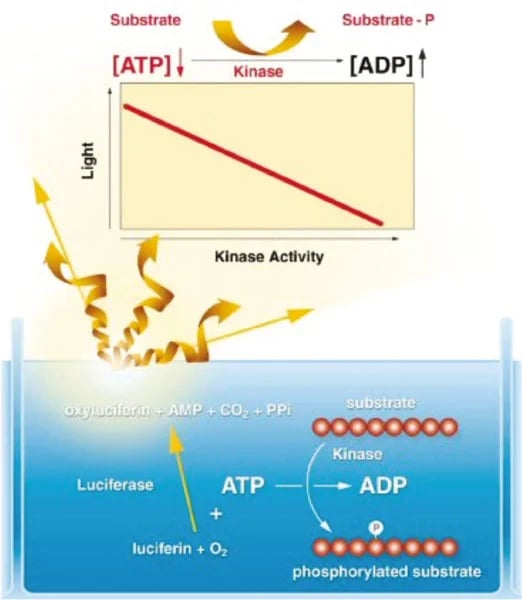
During a kinase reaction, the level of free ATP in the reaction mixture decreases as the γ-phosphate is transferred from the ATP molecule to the kinase substrate. This drop in free ATP can then be accurately measured. The bioluminescent reaction is catalysed by the firefly luciferase enzyme and provides speed, sensitivity, and convenience. The reagent contains Luciferin and Luciferase, which emits a stable light signal, the intensity of which is proportional to the concentration of ATP (figure 1).
Assay Principle: The amount of free ATP added to the reaction mixture which is consumed during the kinase reaction can be accurately measured using the patented bioluminescent Luciferase kinase reagents from Lonza. A stable light signal is emitted that has an intensity which is proportional to the concentration of ATP present. As the kinase reaction progresses the ATP concentration drops and the light emitted becomes less intense.
Materials & Methods
All materials were obtained through normal distribution channels from the manufacturer stated.
- Lonza PKLightTM kit
- PHERAstar FS, BMG LABTECH, Germany
- Microplates, white 384-well, Greiner, Germany
- ATP, BSA, Sigma UK
- PKA, Kemptide, H-89 dihydrochloride, Calbiochem®
The Lonza Kinase Assay reagents were used to investigate the inhibition by H-89 dihydrochloride of cAMP dependant protein kinase (PKA) phosphorylation of Kemptide substrate. Enzyme, Substrate, ATP, and H-89 dihydrochloride were diluted to working concentrations using the same PKA assay buffer consisting of 40 mM Tris-HCl (pH 7.5); 20 mM MgCl2, and 0.1 mg/mL BSA in purified water.
ATP Detection
ATP was diluted in PKA assay buffer to give a concentration range of 0 – 12.5 μM and added into a Greiner 384-well plate. ATP detection reagent was added and read after 1 minute using a 1 second integration time in luminescence mode on the PHERAstar FS.
PKA Activity/Inhibition
Using white 384-well plates, 5 μL of PKA, 5 μL Kemptide, 5 μL of ATP, and 5 μL of Inhibitor (H-89 dihydrochloride) or PKA assay buffer was added to each well. Giving a total assay volume of 20 μL per well. The reaction mixture was incubated for 20 minutes at room temperature.
The remaining amount of ATP was determined by adding 10 μL of the ATP detection reagent to the well and incubating for 10 minutes at room temperature. The 384-well plate was read on the PHERAstar FS in luminescence mode using 1 second integration.
Results & Discussion
The PKLightTM Protein Kinase Assay as shown in figure 2 can detect low levels of ATP and has exceptional linearity (R2 >0.99) over the range used on the PHERAstar FS.
As shown in figures 3 and 4 the PKLightTM assay can accurately determine kinase activity and inhibition, giving reliable IC50 data and clean hits.
Conclusion
Using the cAMP Dependant Protein Kinase (PKA) and Kemptide substrate as a model we have demonstrated the usefulness of using the Lonza kinase reagents to measure kinase activity and inhibition.
This method is very simple to develop and run and can potentially be applied to any ATP-dependent protein kinase and target substrate. We have proved the assay to be robust with Z’ values greater than 0.8 exhibited and reproducible and sensitive enough to determine low potency inhibition. The assay can be easily miniaturised down to 384-well plate formats with the potential to go beyond.