Introduction
Bacteria can monitor and respond to changes in environmental conditions via a cell density process known as quorum sensing (QS). Bacteria use this process to monitor their community by producing, detecting, and responding to low molecular mass signal molecules, called autoinducers (AI). When the cell density increases the concentration of these signaling molecules will also increase. Once the accumulation of these molecules reaches a threshold, bacteria are collectively able to regulate gene expression and therefore cooperatively regulate their metabolic behavior. Quorum sensing has been shown to regulate a variety of processes in bacteria. These include bioluminescence and symbiosis in Vibrio fischeri, expression of virulence genes in Pseudomonas aeruginosa, expression of virulence, surface proteins, and biofilm formation in Escherichia coli, and biosynthesis of extracellular polymeric substances and pathogenicity in Erwinia stewartii. Two major types of quorum sensing molecules (QSMs) have been widely described in literature. The commonest QSM used by Gram-negative bacteria are known as N-acyl homoserine lactones (HSLs) whilst Gram-positive bacteria use amino acids and short peptides (oligopeptide) as their AIs.
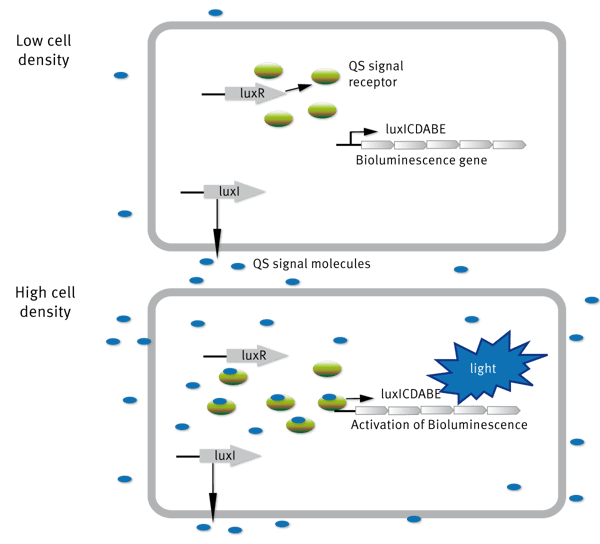
Quorum sensing was first described in V. fischeri, a Gram-negative bacteria. At low cell density, Vibrio fischeri is non-bioluminescent, but when the concentration increases (high cell density), the organism is bioluminescent. The molecular basis for regulation of bioluminescence in V. fischeri via quorum sensing has been well studied. The gene cluster responsible for light production consists of eight genes (luxA-E, luxG, luxI, and luxR) (Figure 1).
The regulator proteins responsible for quorum sensing in this organism are proteins encoded by luxI and luxR. LuxI encodes the enzyme AHL synthase, which catalyzes the reaction involved in the biosynthesis of HSLs known as N-3-oxo-hexanoyl-L-homoserine lactone (3-oxo-C6-HSL). LuxR encodes the protein which binds to the AI and also activates the luxA-E and luxI operons.
The aim of this study was to elucidate the effect of changes in environmental conditions such as growth media as well as the addition of exogenous homoserine lactone on 3-oxo-C6-HSL on the growth and bio-luminescence in V. fischeri. A BMG LABTECH microplate reader was used to measure absorbance and luminescence in script mode.
Materials & Methods
Bacteria strain and Media
V. fischeri ESR1 and its mutants were kindly supplied by Prof Edward G. Ruby. The ESR1 variants include a signal-negative mutant that does not synthesize the 3-oxo-C6-HSL signal but can still respond to exogenous 3-oxo-C6-HSL, (V. fischeri KV240) and a signal-blind strain that produces 3-oxo-C6-HSL but does not respond to 3-oxo-C6-HSL (V. fischeri KV267).
All V. fischeri strains were grown in Luria-Bertani salt media (LBS), which contains 1% (wt/vol) tryptone, 0.5%(wt/vol) yeast extract (wt/vol), 2% NaCl (wt/vol), and 0.3% (vol/vol) glycerol in 50 mM Tris-HCl (pH 7.5) or in seawater tryptone (SWT) which contains 0.5% (wt/vol) tryptone, 0.3% (wt/vol) yeast extract, and 0.3% glycerol (vol/vol) in 70% seawater.
Evaluation of V. fischeri growth and bioluminescence under different media and concentrations of homoserine lactone
The growth and bioluminescence of all V. fischeri strains were monitored using a multi-mode plate reader from BMG LABTECH. Briefly, overnight cultures of V. fischeri strains grown in the above media at 28°C were inoculated to fresh media (1:500 dilution) with or without the addition of exogenous 3-oxo-C6-HSL. 200 μL of diluted cells (quadruplicate) were then transferred into a white 96 well clear bottom microplate (Greiner Bio-One).
The instrument incubation temperature was set at 28°C with continuous shaking at 300 rpm. The OD600 and luminescence were then measured every hour for at least 16 h. The V. fischeri bioluminescence reading was reported as relative light units (RLU) divided by OD600.
Results & Discussion
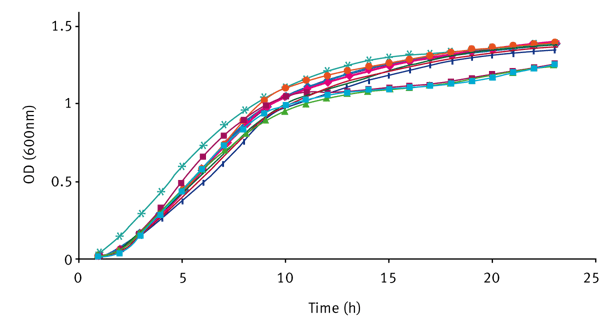
The increase in cell number over time for V. fischeri strains, grown in LBS and SWT can be seen in figure 2. The growth curves represent a typical growth curve under batch conditions with two clearly distinct phases i.e. exponential 2-8 h and the onset of stationary phase after 8 h.
There was no significant difference between each strain, suggesting that quorum sensing in V. fischeri does not promote or inhibit growth.
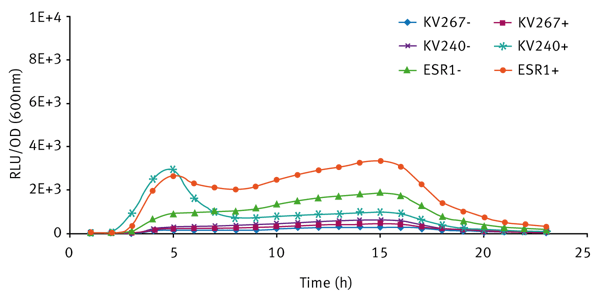
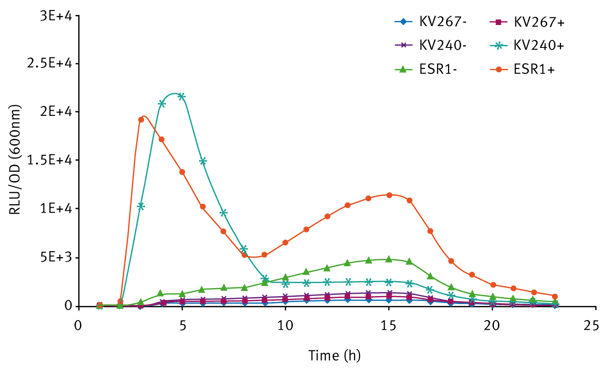
The expression of bioluminescence by V. fischeri ESR1 cultivated in LBS and SWT (with or without 3-oxo-C6-HSL) can be seen in Figures 3 and 4 respectively. For V. fischeri ESR1, bioluminescence was higher in SWT than in LBS. Earlier activation of bioluminescence was observed when exogenous 3-oxo-C6-HSL was added to V. fischeri ESR1 at the onset of growth. For V. fischeri KV240, bioluminescence was only observed when exogenous 3-oxo-C6-HSL was added at the onset of growth. This confirms that the strain lacks the ability to produce endogenous 3-oxo-C6-HSL and can only express bioluminescence upon the addition of exogenous 3-oxo-C6-HSL. As expected V. fischeri KV267, did express bioluminescence with or without the addition of exogenous 3-oxo-C6-HSL since it lacks the ability to respond to the signal molecule.
Conclusion
In this study, we show that BMG LABTECH microplate readers are a useful tool for understanding quorum sensing in bacteria. The instrument is able to monitor microbial growth and bioluminescence in parallel.