Introduction
A major pathological hallmark of Parkinson’s disease (PD) is the presence of α-Synuclein (αS) amyloid fibrils within cytotoxic Lewy bodies, in the brain. The cryo-EM structure of αS (a 14 kDa protein) is known and consists of three distinct regions: a central nonamyloidogenic component (NAC, residues 61–95); an N-terminal region (NTR, residues 1–60) and C-terminal region (residues 96–140). The NAC region of αS is largely hydrophobic and prone to aggregation by acting as a ‘seed template’ for αS fibrilization. In addition, a recent study identified a specific sequence motif within the NTR region (residues 36–42) to be crucial to amyloid fibril formation.1
Here, we have designed and synthesized four isomeric (L- and D-isomeric) peptides as potential inhibitors of αS aggregation.2 These peptides are based on two short sequences found in the NAC (residues 77-82) and NTR (residues 36-42) regions of αS and were termed, NTRTP-L, NTR-TP-D, NAC-TP-L, and NAC-TP-D, respectively. The amino acid sequence of peptides is: GVLYVGS-Aib (NTR-TP-L), gvlyvgs-Aib (NTR-TP-D), VAQKTV-Aib (NACTP-L) and vaqktv-Aib (NAC-TP-D). They were designed to recognize and incorporate themselves into the growing β-sheet structure of αS, with the C-terminal α-aminobutyric
acid (Aib) residue functioning as a β-strand breaker.
Assay principle
The αS elongation assay and primary nucleation assay are two well-established fluorescence techniques. Both assays monitor increased Thioflavin T (ThT) fluorescence, as purified wild-type αS slowly aggregates from solution with prolonged shaking. ThT can incorporate itself, from the buffer, into the growing insoluble β-sheet structure of αS leading to an increase in fluorescence directly correlated to cross β-sheet formation. For the elongation assay, small
seed fibrils of aggregated αS are also added to wild type αS at the start of the reaction. This substantially decreases reaction time (24 h) as compared to the primary nucleation assay (144 h). 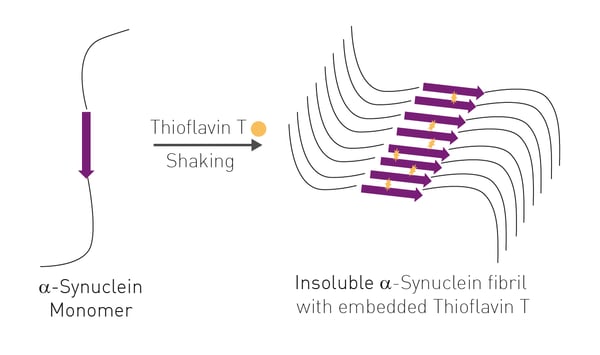
- 384-well microplate (black polystyrene, Greiner)
- Thioflavin T (Sigma-Aldrich)
- Monomeric αS wild-type (WT)
- NAC and NTR, L & D peptidic inhibitors
- Omega series plate reader (BMG LABTECH)
Experimental procedure
Expression and purification of monomeric αS wild-type (WT) was performed as previously described.3 Peptidic inhibitors were synthesised via solid phase peptide synthesis using Fmoc chemistry.2 Seed fibrils for elongation assays were obtained by heating and stirring monomeric αS (50 μM in PBS, pH 7.4) at 45 °C for 24 h followed by sonication. The ability of NAC and NTR peptides to prevent αS fibril formation was assessed using either an αS elongation assay or a αS primary nucleation assay. Elongation of αS fibrils was monitored using ThT (20 μM) for 24 h at 37 °C. Peptide inhibitors were added at 1:1 and 1:5 M ratios (αS:peptide) to monomeric αS (50 μM in PBS, pH 7.4) with 5% (w/w) αS seed fi brils. Primary nucleation-mediated fibril formation of αS was also monitored using ThT (20 μM) for 144 h at 37 °C. Peptide inhibitors were added at 5:1, 1:1 and 1:5 M ratios to monomeric αS (100 μM in PBS, pH 7.4). All assays were performed at least three times and data are reported as mean ± SEM of these independent assays.
Instrument settings
|
Optic settings
|
Fluorescence, plate mode kinetic
|
|
|
Filter
|
Ex 440-10 |
|
|
General settings
|
Number of flashes | 50 |
| Settling time | 0.5 sec | |
|
Kinetic settings
|
||
|
Elongation assay
|
Number of cycles
|
91
|
|
Cycle time
|
1800 s
|
|
|
Nucleation
assay |
Number of cycles
|
250
|
|
Cycle time
|
1750 s
|
|
|
Incubation
|
37°C
|
|
|
Shaking
|
20 s before start of each cycle
|
|
|
Elongation assay: 120 rpm double orbital
|
||
|
Nucleation assay: 300 rpm double orbital
|
||
Results & Discussion
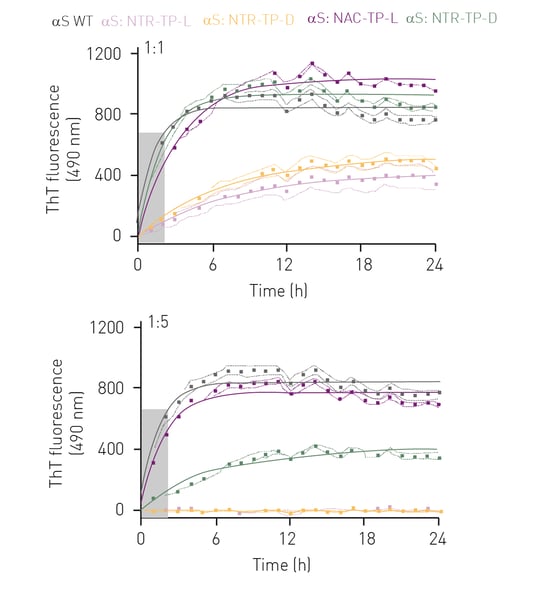
In the fibril elongation assay αS exhibited one-phase association-like kinetics typical of αS fibril elongation, where a fluorescence plateau was observed after 4 h, indicative of mature fibril formation (figure 2, grey curve).
At a 1:1 molar ratio, both L- and D-isoforms (figure 2, purple and green curves) of the NAC-TPs did not prevent αS fibril formation nor reduce the rate of fibril elongation. However, both NTR-TPs drastically reduced fibril formation (47% inhibition, L-isoform and 31% inhibition, D-isoform) and rate of elongation (8-fold decrease, L-isoform and 9-fold decrease, D-isoform) (fi gure 2, rose and yellow curves). Interestingly, at a 1:5 M of αS:peptide, the NAC-TP D-isoform greatly reduced αS fibril formation by 51%, as well as the rate of fibril elongation (figure 2, green curve). In comparison, NAC-TP L was ineffective in altering αS elongation kinetics and preventing mature fibril formation. Significantly, both NTR-TPs effectively prevented fibril elongation (15-fold reduction) and formation (98% inhibition).
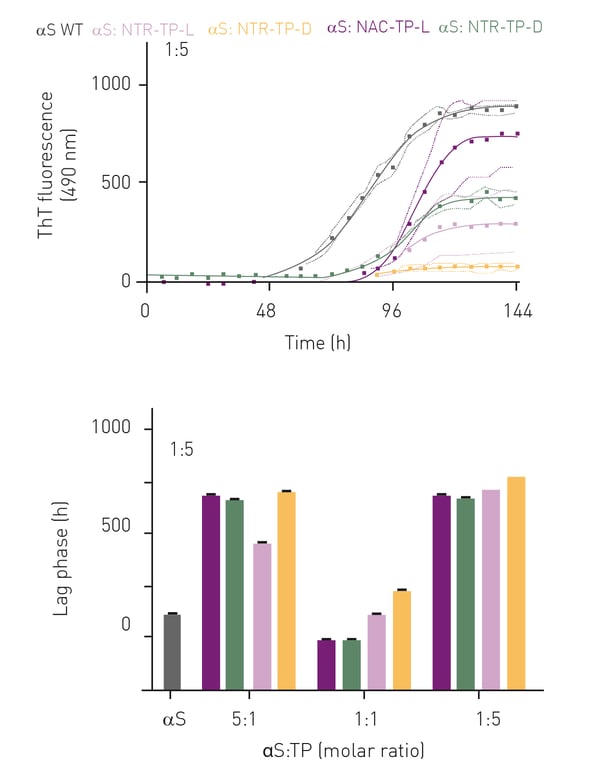
Primary nucleation assays were performed with 1:1, 1:5 and 5:1 ratios of αS:inhibitor mixtures. With no inhibitors added, αS exhibited sigmoidal-like aggregation kinetics whereby the lag-phase lasted for 28 h, with signifi cant fibril elongation observed after 52 h and reaching a plateau at 115 h (figure 3A, grey curve). In the presence of NAC- and NTR-TPs, we observed sigmoidal-like aggregation kinetics across all molar ratios (figure 3, upper panel, only 1:5 M is shown).
Collectively, we observed a decrease in total fibril yield with increasing concentrations of NAC- and NTR-TPs (data not shown). In addition, the presence of
either NAC- or NTR-TPs increased the lag-phase across all molar ratios examined, except for the NAC-TPs (both L-and D-isoforms) at a 1:1 M ratio where the lag-phase was reduced compared to αS alone (figure 3, lower panel)
Conclusion
Overall, we propose that NAC- and NTR-TPs both decrease the rate of nucleation and prevent fibril elongation, thus reducing αS fibril yield. Cell viability studies also showed a significant reduction of αS aggregate-induced cytotoxicity on Neuro-2a and Caco-2 cells for both NAC- and NTR-TPs2. Collectively, study lays the foundation for further design and development of drug candidates in the treatment of PD.
The work also highlights the eminent suitability of BMG plate readers for ThT aggregation assays. The Optima (the machine used in this study is >15 years old and still going strong) has been superseded by the Omega plate reader. The Omega is equipped with a heavy-duty shaking mechanism, heating and software that allows easy setup of controlled shaking algorithms for all types of ThT aggregation assays..
References
-
Doherty CPA, et.al., A short motif in the N-terminal region of α-synuclein is critical for both aggregation and function, Nat. Struct. Mol. Biol. 27 (2020) 249–259.
-
Horsley JR, et. al., Designer D-peptides targeting the N-terminal region of α-synuclein to prevent parkinsonian-associated fibrilization and cytotoxicity; BBA - Proteins and Proteomics 1870 (2022) 140826.
-
Buell AK, et. al., Solution conditions determine the relative importance of nucleation and growth processes in alpha- synuclein aggregation, Proc. Natl. Acad. Sci. U. S. A. 111 (2014)
7671–7676.