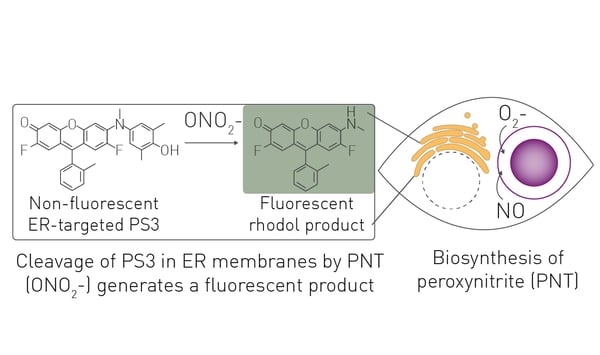
Introduction
The tumor microenvironment (TME) consists of a variety of immune cells, blood vessels, and other cells and molecules that surround primary cancer cells1. The interplay between cancer cells and the TME has taken on increasing importance as our understanding of the pro-tumor and anti-tumor effects of immune and other cells have come to light. As the TME is studied more extensively, it has become clear that there is much to learn about this complex
system and its interplay with cancer at various stages of progression. This knowledge benefits the development of new therapeutics for treatment of a wide variety of cancers.
Myeloid derived suppressor cells (MDSCs) proliferate extensively in the TME of many cancer patients2. These cells contribute to immunosuppression and protect cancers from the immune system in several ways. One way is by generating the reactive oxidant peroxynitrite within the TME, which leads to destructive nitration of protein tyrosine residues. The effects of peroxynitrite within the TME are not yet fully understood, in part, due to a lack of sufficiently
sensitive assays to detect this short-lived compound.
Here, we describe an assay that uses the optimized small molecule sensor termed peroxynitrite sensor 3 (PS3) that becomes fluorescent after cleavage by peroxynitrite.3,4
Assay principle
- TentaGel M NH2 microspheres (10 μm, Rapp Polymere)
- DNP-NHS (MilliporeSigma)
- Rabbit anti-DNP IgG (Vector Laboratories)
- 96-well, untreated plates (USA Scientific)
- CLARIOstar Plus (BMG LABTECH)
Experimental procedure
Opsonized IgG/beads and DNP-only control beads were prepared using microspheres, DNP-NHS and anti-DNP IgG as previously described2. RAW264.7 macrophages and the MSC2 MDSC-like cell line were cultured as described2. For fluorescent detection of peroxynitrite in cells, cells were seeded at 40K/well. After 16 h, the PS3 sensor was prepared as described2. Following treatment with PS3 and immunostimulatory opsonized beads and controls,
cells were incubated for 4 hours at 37 °C before detection of fluorescence with the CLARIOstar Plus. Similar fluorescent assays were prepared for MDSCs
isolated from mice and humans2.
Instrument settings
|
Optic settings
|
Fluorescence, endpoint
|
|
|
Monochromator
settings |
Ex 484-15 |
|
|
Gain
|
850 | |
|
Focal height
|
Adjusted pre-run |
|
|
General settings
|
Optic used | Top |
| Flashes | 20 | |
Results & Discussion
The fluorescent sensor PS3 was previously validated as capable of detecting production of peroxynitrite induced by antibody-dependent cellular phagocytosis in several phagocytic cell lines3,4. MDSCs have also been described as phagocytic. Therefore, the first step was to determine if stimulation of antibody-dependent cellular phagocytosis in MDSCs causes an increased fluorescent signal from the PS3 sensor.
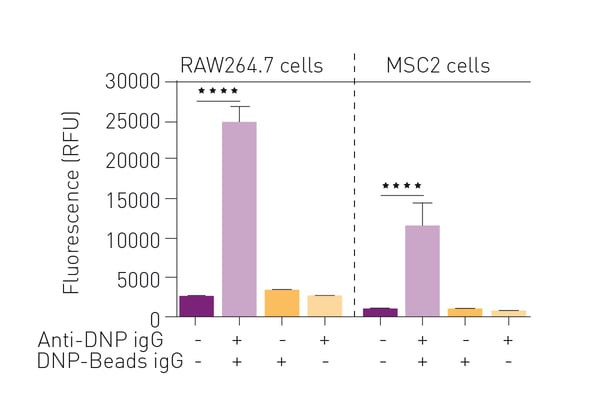
As shown in figure 2, the RAW264.7 macrophage cell line showed fluorescence only when conditions for antibodydependent cellular phagocytosis were met. Similarly, the MDSC-like cell line MSC2 exhibited increased fluorescence under conditions where phagocytosis was stimulated. The robust fluorescent signal detected in MSC2 cells encouraged evaluation of this assay of PNT in mouse models of cancer and human cancer patients where MDSC expression was expanded.
 Using a mouse mammary carcinoma model based on injection of EMT6 cells, it was found that splenocytes from tumor bearing animals exhibit high fluorescence in the peroxynitrite detection assay compared to splenocytes from control animals. Furthermore, MDSCs isolated from splenocytes of mice with tumors also exhibit high fluorescence in the assay2. As shown in figure 3, purification of MDSC sub-types using FACS revealed that granulocytic, but not monocytic, MDSCs are responsible for the production of peroxynitrite. Finally, PS3 was tested using samples from human melanoma patients and
Using a mouse mammary carcinoma model based on injection of EMT6 cells, it was found that splenocytes from tumor bearing animals exhibit high fluorescence in the peroxynitrite detection assay compared to splenocytes from control animals. Furthermore, MDSCs isolated from splenocytes of mice with tumors also exhibit high fluorescence in the assay2. As shown in figure 3, purification of MDSC sub-types using FACS revealed that granulocytic, but not monocytic, MDSCs are responsible for the production of peroxynitrite. Finally, PS3 was tested using samples from human melanoma patients and
healthy human volunteers.
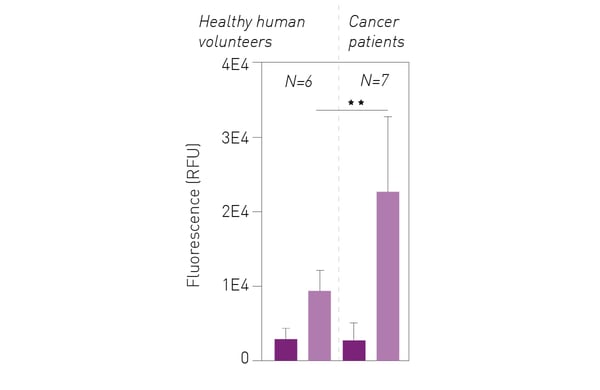
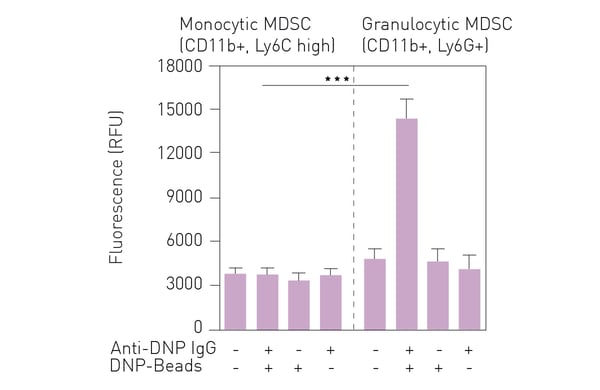 Figure 4 shows that peripheral blood mononuclear cells derived from melanoma patients exhibited a much higher fluorescence in the peroxynitrite assay than those from healthy donors. Peroxynitrite production remained dependent on induction of antibody-dependent cellular phagocytosis.
Figure 4 shows that peripheral blood mononuclear cells derived from melanoma patients exhibited a much higher fluorescence in the peroxynitrite assay than those from healthy donors. Peroxynitrite production remained dependent on induction of antibody-dependent cellular phagocytosis.
Conclusion
We show proof of principle for a microplate reader-based assay that can detect peroxynitrite produced during antibody-dependent cellular phagocytosis using the PS3 fluorescent sensor. Due to the role of peroxynitrite in affecting cell function in the tumor microenvironment, this assay provides a useful tool for better understanding cancer progression and may ultimately assist with the identification of improved treatments that overcome immunosuppression in cancer.
References
-
de Visser, KE & Joyce, JA. The Evolving Tumor Microenvironment. Cancer Cell (2023) 41: 347-403.
-
Carlson, EJ, et al. Fluorescent Detection of Peroxynitrite Produced by Myeloid Derived Suppressor Cells in Cancer and Inhibition by Dasatinib. ACS Pharmacol Transl Sci (2023) 6: 738-747.
-
Rane, D et al. Fluorescent Detection of Peroxynitrite During Antibody-Dependent Cellular Phagocytosis. Methods Enzymol (2020) 640: 1-35.
-
Knewtson, K et al. Targeting Fluorescent Sensors to Endoplasmic Reticulum Membranes Enables Detection of Peroxynitrite During Cellular Phagocytosis. ACS. Chem. Biol. (2018) 13: 2595-2602.