Introduction
Respiratory diseases such as cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) involve cycles of chronic infection and prolonged inflammation of the airways, leading to poor lung function. Antibiotics and anti-inflammatory treatments have been shown to help patients in these cases.1 In both CF and COPD, neutrophils drive the inflammatory response,2,3 and the presence of neutrophil elastase (NE), a protease secreted by neutrophils, has been established as a key biomarker of infection and inflammation.
The detection of NE can therefore be used to monitor the disease status of the patient as it correlates with the occurrence of acute episodes of disease activity.
Established methods for the quantitation of active proteases are based on chromogenic or fluorogenic peptides that, in complex biological samples (such as sputum), can be broken down by other activities (either host or bacterial in origin) and therefore lack the necessary specificity for accurate quantitation. Traditional immunoassays do not distinguish between the active and inactive forms of the enzyme thus do not accurately map the severity of disease.
In this application note, the Active NE ProteaseTag™ kit is shown to detect and quantify levels of NE in patient samples. ProAxsis’ ProteaseTags™ are engineered to inhibit and bind to specific proteases. Once bound, they form a bridge to a solid support allowing their incorporation into an immunoassay format for specific quantification.
Assay Principle
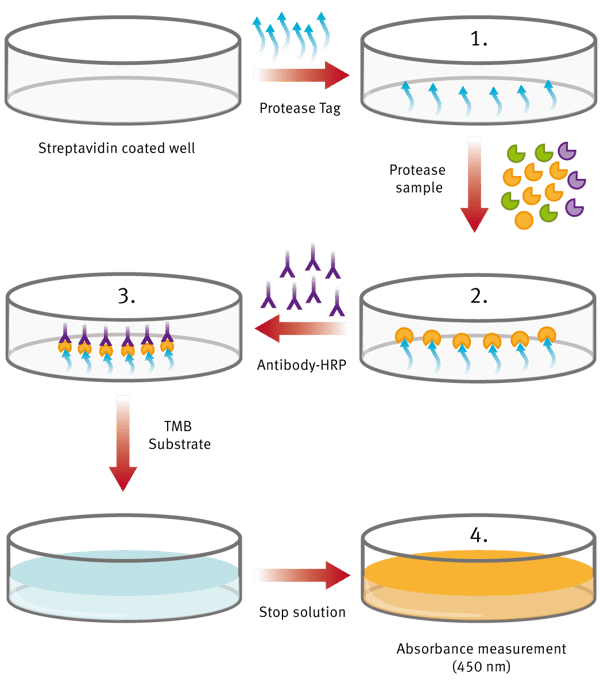
The ProteaseTag™ Active NE Immunoassay captures active NE using NE-Tag. (Fig.1) A subsequent antibody step provides additional signal amplification with increased sensitivity. Detection is achieved within an ELISA-type format and provides results in less than 3 hours. Detection with NE-Tag is quantitative and discriminatory. The assay kit is suitable for the measurement of the following:
- sputum sol
- bronchoalveolar lavage
- wound exudate
- serum free cell culture
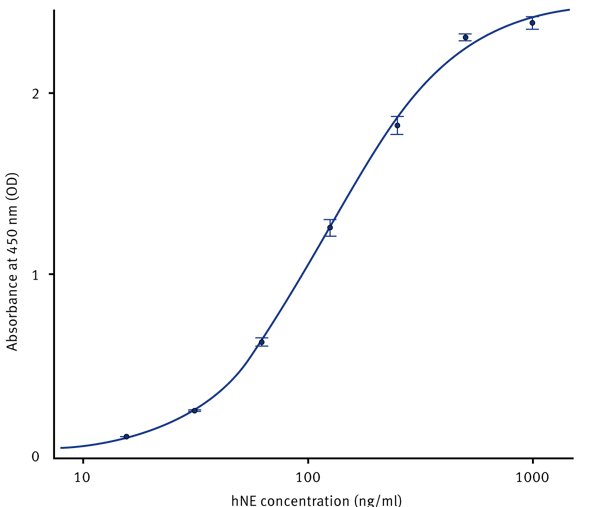
The detection range of the assay is 1000-15.6 ng/mL and a sensitivity of 3.3 ng/mL.
Summary of the procedure
1. Coat plate with NE-Tag. Wash.
2. Add standards and samples. NE-Tag captures active NE from the sample, inactivating and stabilizing the enzyme. Wash.
3. An antibody-conjugate step enables detection of the complex by the subsequent addition of colorimetric substrate/stop solution.
4. Measure the plate in absorbance mode at 450 nm.
Materials & Methods
- ProteaseTag™ Active Neutrophil Elastase Immunoassay (PA001) from ProAxsis
- CLARIOstar microplate reader from BMG LABTECH
Prior to the assay, sputum samples were collected from individuals with CF and briefly processed. All standards and samples were kept on ice before addition to the microplate well. All other reagents were brought to room temperature before addition.
Standards were diluted and the plate prepared according to the manufacturer´s instructions and samples, standards and blanks added to the plate in duplicate.
Instrument settings
| Measurement Type: | Absorbance |
| Reading Mode: | Endpoint |
| Wavelength: | Spectra or Discrete wavelength at 450 nm |
| Settling time: | 0.5 sec |
| Shaking settings: | Shaking frequency [rpm]: 300 |
| Shaking mode: double orbital | |
| Shaking timing: 3 sec before plate reading | |
| Temperature: | 37°C |
Results & Discussion
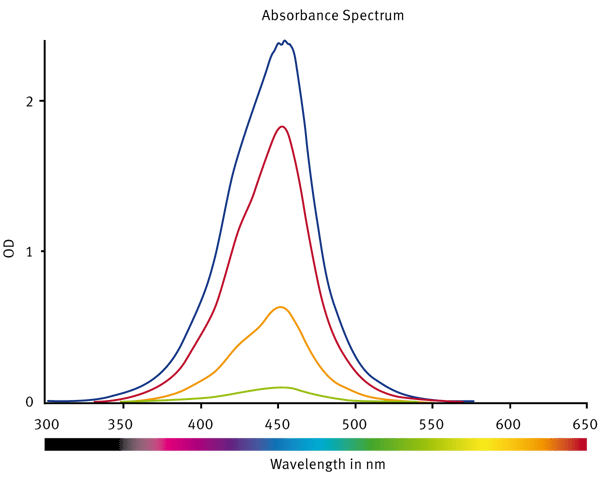
First, the spectral profile of the standards was analysed (Fig. 2). This confirmed that 450 nm was the optimum wavelength at which to read the absorbance.
Initial raw data was blank corrected using the MARS Data Analysis software. A standard curve was generated with the help of the standard curve wizard. The hNE concentration from each sample was then automatically calculated from the regression curve (Fig. 3).
Conclusion
The ProteaseTag™ Active NE Immunoassay provides a simple and quick method of assessing active neutrophil elastase levels in a given sample. Because the assay accurately quantifies active protease levels, the kit constitutes a reliable method for the detection of infection and inflammation in clinical samples. In addition to airways samples, active NE has been quantified in a number of other biological samples such as wound fluid. The kit significantly correlates with other activity-based methods for the detection of active NE but has greater selectivity and specificity than peptide-based substrates when used with complex biological samples. This can be of significant impact when determining relationships or associations between a protease biomarker and other markers of disease.
The CLARIOstar provides a platform on which to read and analyse data to completion, resulting in a fast and easily reproducible method for running the assay.
References
- Elizur, A. et al. (2008) Chest. 133(2), 489-495.
- Gifford, A.M., Chalmers, J.D. (2014) Curr. Opin. Hematol. 21(1), 16-22.
- Tetley, T.D. (2005) Curr. Drug Targets Inflamm. Allergy. 4(6), 607-618.