Introduction
Molecular oxygen is a key substrate of all aerobic organisms and the terminal acceptor of the electron transport chain (ETC). This highly informative marker of cellular metabolism and mitochondrial function is closely regulated by the ATP/ADP ratio, the levels of available oxygen, and the action of signalling molecules such as NO and Ca2+. Analysis of molecular oxygen facilitates the elucidation of critical biochemical pathways; including cell survival and death, mitochondrial function, toxicological impact of compounds, and metabolic alterations caused by various stimuli or disease states. To date, routine analysis of intracellular molecular oxygen has been limited by the lack of suitable means of sensing oxygen within the cell monolayer.
Assay principle
MitoXpress Intra is an oxygen-sensitive probe developed for the intracellular analysis of molecular oxygen using plate-based time-resolved fluorometry. Measurement is based on the ability of O2 to quench the emission of a probe which is taken up by endocytosis. As cells respire, the concentration of oxygen within the cell monolayer is depleted and this depletion is seen as an increase in probe signal, expressed as probe lifetime, following ratiometric measurement of time-resolved phosphorescence intensity. This allows real-time information on the concentration of molecular oxygen within the cell monolayer to be generated across multiple samples without a requirement for specialised imaging equipment. The ability to monitor such fluctuations is particularly informative as it allows the assessment of transient changes in metabolic activity and provides access to a critical parameter in the study of hypoxia which is beyond the capacity of extracellular sensing methodologies.
Materials & Methods
- MitoXpress Intra Intracelluar Oxygen Assay from Agilent Technologies (MX-300-4)
- BMG LABTECH microplate reader equipped with Atmospheric Control Unit (ACU)
Probe preparation and cell loading
Cells are grown to full confluence in a suitable medium supplemented with foetal bovine serum, L-glutamine, and Penicillin/ Streptomycin on cell culture treated 96 well plates. Replace seeding medium with 100 µl of normal growth media (10% FBS) containing MitoXpress Intra probe at 10µg/ml and return to culture overnight. Prior to measurement, replace media with 150 µl of pre-warmed media.
Measurement
Intracellular oxygen in samples is measured using the BMG LABTECH microplate reader and the scripting function. Optimal filter wavelengths are 340 TR L for excitation and EM 655-50 for emission in Omega readers and EM 645-20 for CLARIOstar. Phosphorescent intensities are measured at delay times of 30 µs and 70 µs (with 30 µs window time) with the ratio of these intensities subsequently converted to phosphorescent lifetimes as shown in Figure 1. These conversions are performed with the help of the MARS data analysis software.

Results & Discussion
Typical Data Output
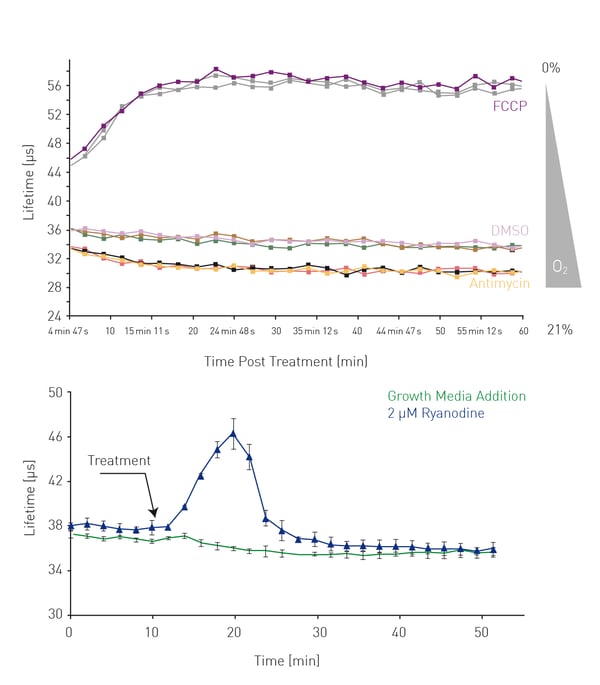
When analysing a fully confluent monolayer, ETC activity results in measurement usually beginning at oxygen concentrations below air saturated levels. Reduced ETC activity results in an increase in intra-cellular oxygen concentration resulting in a decrease in probe signal while increased ETC activity results in a decrease in intracellular oxygen concentration resulting in an increase in probe signal (Fig 2). Increases may be prolonged or more transient, depending on the processes involved and the measurement conditions.
Probe Response
Measurement of phosphorescent lifetime of the MitoXpress Intra probe facilitates measurement of the basal level of intracellular oxygen within the cell monolayer. The response of the probe is demonstrated in Antimycin treated HepG2 cells measured on a microplate reader equipped with an atmospheric control unit (BMG LABTECH). This instrument allows the ambient O2 concentration to be reduced stepwise. The time taken for the non-respiring monolayer to equilibrate to this new O2 concentration can be observed (Fig. 3) with the new steady state probe signal reflecting this new concentration. Such calibrations also allow measured lifetime values to be automatically transposed into O2 concentrations, thereby allowing probe response to be viewed in this scale.
Assay Performance & Cell Responses
Figure 4A shows the effect of HepG2 cells treated with an electron transport chain inhibitor (Antimycin) and uncoupler (FCCP). The magnitude, consistency of monolayer (de)oxygenation is monitored. Profiles are seen to be highly consistent with uncoupling causing enhanced oxygen consumption seen by an immediate decrease in intracellular oxygen resulting in an increase in probe lifetime while inhibition causes the reverse. Increasing the FCCP concentration increases the speed of the deoxygenation while increasing the cell number increases the depth of the deoxygenation (data not shown). Treatment of HCT116 cells with the RyR1 agonist ryaniodine also causes a transient de-oxygenation of the monolayer via a calcium-mediated increase in metabolic rate (Fig. 4B), a response not readily apparent using extracellular analysis.
Conclusion
MitoXpress Intra allows the convenient monitoring of intracellular oxygen on a conventional microtitre plate format. The probe is ‘self loading’ and provides excellent signal to blank performance. This allows the investigation of a variety of parameters currently beyond the capabilities of existing probe technologies.