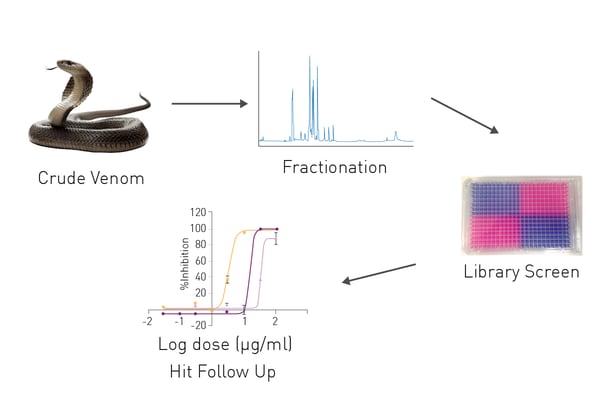
Introduction
Canine mammary tumors are the most common type of cancer in intact female dogs; with approximately 50% of them being diagnosed as malignant1,2. Commonly used treatments for human breast cancer, including chemotherapeutic drugs have been reported to be ineffectual in canine tumors. Animal venom contains a complex mixture of proteins, peptides, enzymes, and small molecules. In addition to their negative physiological effects on human health, components from venoms have been utilised as treatments for conditions such as hypertension, angina and even cancer3. In this study, venoms were tested for their specific cytotoxic effect on canine mammary cancer cells with a resazurin viability assay.
Assay principle
Resazurin salt is a cell viability reagent that, unlike MTT, allows for the development of both a fluorescent and colorimetric kinetic assay. Resazurin is an oxidation-reduction (REDOX) indicator that can be readily used in assays to assess cellular viability. 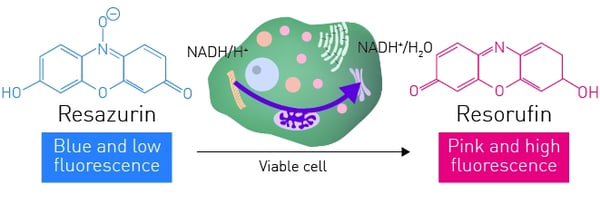
-
384-well microplate, clear, flat-bottom, TC-treated (Falcon, #353961)
-
RPMI media, Fetal-calf serum, Penicillin-streptomycin, L-glutamine (Fisher Scientific)
-
Resazurin salt (Sigma Aldrich)
-
CLARIOstar Plus microplate reader (BMG LABTECH)
Experimental procedure
Assay optimisation and confirmation was undertaken as followed. Canine mammary tumour (CMM26) cells were plated into clear 384-well plates at 2x104 cells/well. Cells were either treated with a lethal dose of cobra venom for 2 h or left untreated. After 2 h, all media was removed and replaced with 25 μl of 160 μM resazurin/RPMI media mix. Conversion of resazurin to resorufin was monitored over time (excitation 544 nm, emission 590 nm) using a CLARIOstar Plus plate reader and Z’ values calculated for the assay at 15 min intervals up to 300 min post resazurin addition.
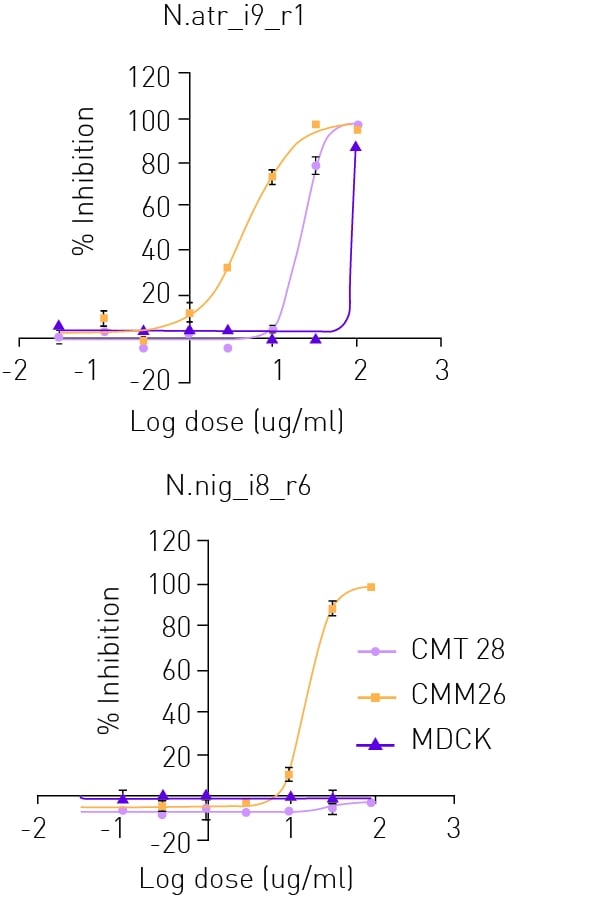
For the pre-selection of venom fractions, a Cytotoxic Targeted Venom Discovery Array™ (T-VDActx) containing two dimensional HPLC fractions from twelve diverse venomous species was screened against CMM26 cells seeded as previously described. Cells were dosed with venom fractions in supplemented RPMI media at a final working concentration of 35 μg/ml for 2 h. Cell viability was measured as previously determined. CMT28 and CMM26 canine mammary cancer cells and MDCK canine kidney cells, were seeded into 384-well clear bottom plates and cultured in a humidified incubator overnight at 37 °C, 5% CO2. Based on the pre-selection, cells were dosed with purified venom fractions in a ½ log dose response (μg/ml) for 2 h (Fig. 2). After 2 h venom fractions were removed and resazurin development and detection undertaken as previously described.
Instrument settings
|
Optic settings
|
Fluorescence, plate mode kinetic
|
|
|
Filter
|
Ex 544 |
|
|
General settings
|
Number of flashes | 10 |
| Settling time | 0.1 s | |
|
Kinetic settings
|
Number of cycles
|
68
|
|
Cycle time
|
900 s
|
|
|
Incubation
|
37°C
|
|
Results & Discussion
Z’ analysis of the resazurin assay (Fig. 3) demonstrated that the assay was robust from timepoint 15 min of incubation onwards with a maximum achievable Z’ value of 0.74.
Of the 535 T-VDA fractions screened, 13 fractions were found to cause ≥70% inhibition and all 13 were found to have a statistically significant effect (One-way ANOVA with Tukey’s post-test, P<0.05) compared to the untreated control cells in CMM26 cells, showing a hit rate of 2%.
Hit fractions were confirmed in dose-response mode (n=3 +/- SD) (see Fig. 4) against CMM26 (yellow line), additional canine mammary tumour cell line CMT28 (rose line) and normal canine kidney cells MDCK (purple line). All fractions identified in the screening of T-VDActx displayed greater potency against CMM26 than CMT28 canine mammary tumour cells, showing cellular selectivity between the two canine mammary cancer cell lines. Both cancer cell lines showed greater susceptibility than MDCK cells following treatment with all fractions, showing a selective cytotoxicity towards tumour cells over non-tumour cells.
Conclusion
Resazurin fluorescence can be used as a robust and reproducible detection assay method for assessing mammalian cytotoxicity. The assay enables a larger window of detection than tradition MTT assays. In combination with the CLARIOstar Plus cell’s metabolic activity can be monitored in real-time and provides sufficient sensitivity to detect differences in toxicity between cell lines of similar origins.
Screening of TVDActx identified purified fractions that showed specific reductions to canine cancer cell survival over that of non-cancerous canine kidney cells. Some fractions were found to display no toxicity to non-cancerous cells at doses as high as 100 μg/ml. Differential effects in toxicity were also observed between the 2 tested canine mammary tumour cell lines with several of the active fractions, suggesting toxicity is occurring through mechanistic effects rather than general cell cytotoxicity.
References
-
The Ohio State University, Veterinary Medical Centre, https://vet.osu.edu/vmc/companion/our-services/oncology-andhematology/common-tumor-types /canine-mammarytumors
-
Sleeckx, N., de Rooster, H., Veldhuis Kroeze, E. J. B., van Ginneken, C. and van Brantegem, L. (2011) ‘Caninemammary tumours, an Overview’, Reproduction in Domestic Animals, 46(6), pp. 1112–1131. doi: 10.1111/j.1439-0531.2011.01816.x.
-
Calderon, L. A., Sobrinho, J. C., Zaqueo, K. D., et al. (2014) Antitumoral Activity of Snake Venom Proteins: New Trends in Cancer Therapy. BioMed Research International, 2014(203639)