
PHERAstar FSX
Powerful and most sensitive HTS plate reader
Alpha-synuclein is a key protein involved in the onset and progress of neurodegenerative diseases like Parkinson’s. Find out about how microplate readers can help advance alpha-synuclein research.
 Dr Barry Whyte
Dr Barry Whyte
Alpha-synuclein is thought to contribute to the onset and progress of the synucleinopathies, a group of related disorders that includes Parkinson’s disease and dementia with Lewy bodies.1 The involvement of alpha-synuclein in Parkinson’s disease was first proposed around two decades ago based on familial genetic studies.2 However, researchers are still working to find out the precise role that the protein plays in the onset and progression of this degenerative disease.
Around the world, disability and death due to Parkinson’s disease alone are increasing faster than for any other neurological disorder. In 2019, more than 8.5 million individuals were reported globally to have Parkinson’s disease and the prevalence has doubled in the past 25 years (World Health Organization).
In this blog, we look at the relationship between the alpha-synuclein protein and the synucleinopathies. We also discuss how microplate readers can be used in neuroscience to accelerate research into the alpha-synuclein protein.
Alpha-synuclein is a small, disordered protein (140 amino acids) found primarily in the brain. The protein is mainly concentrated in motor regions involved in regulating movement such as the substantia nigra. The physiological function of alpha-synuclein is not completely understood but the protein is believed to play a role in regulating the release of neurotransmitters. In this context, the protein may help to regulate the release of neurotransmitters by binding to vesicles, influencing the movement of these vesicles, and affecting their fusion with the cell membrane of the neuron. Alpha-synuclein has also been linked to other cellular processes such as the regulation of mitochondrial function and response to cellular stress. Certain parts of alpha-synuclein tend to form helical or beta-sheet structures in the protein which can lead to the formation of aggregates or clumps of alpha-synuclein. This aggregation of protein is thought to be one of the early steps in the onset of neurodegenerative disease.
Certain parts of alpha-synuclein tend to form helical or beta-sheet structures in the protein which can lead to the formation of aggregates or clumps of alpha-synuclein. This aggregation of protein is thought to be one of the early steps in the onset of neurodegenerative disease.
The accumulation of abnormal clumps of alpha-synuclein in the brain is one of the defining characteristics of synucleinopathies. The synucleinopathies are however clinically heterogeneous disorders that feature aggregates of alpha-synuclein in neurons and glia in the form of Lewy bodies, Lewy neurites, neuronal cytoplasmic inclusions, and glial cytoplasmic inclusions.3 Overall, they are divided into two major neurodegenerative disease entities: Lewy body disease and multiple system atrophy. Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies are common clinical presentations of Lewy body disease. Multiple system atrophy has two main clinical subtypes: predominant cerebellar ataxia and predominant parkinsonism. A few other rare disorders like neuroaxonal dystrophies have similar features. In Parkinson’s disease, alpha-synuclein aggregates accumulate primarily in the substantia nigra. The accumulation of the protein in this region leads to the degeneration of dopamine-producing neurons, which results in the motor dysfunction often observed in Parkinson’s patients.
In Parkinson’s disease, alpha-synuclein aggregates accumulate primarily in the substantia nigra. The accumulation of the protein in this region leads to the degeneration of dopamine-producing neurons, which results in the motor dysfunction often observed in Parkinson’s patients.
In dementia with Lewy bodies, alpha-synuclein accumulates in areas of the brain involved in memory and thinking, as well as in regions involved in movement regulation. This leads to a combination of symptoms including cognitive impairment, hallucinations, and movement problems. Visually, Lewy bodies are characterized by deposits of alpha-synuclein proteins that appear as spherical masses inside neurons. The German neurologist Friederich Lewy was the first physician-scientist to describe Lewy bodies back in 1912. Some Lewy bodies tend to displace the nucleus to one side of the cell. A typical Lewy body is a cytoplasmic sphere that consists of a dense core surrounded by radiating alpha-amyloid fibrils.
Muscle system atrophy is a less prevalent synucleinopathy in which alpha-synuclein aggregates in several regions of the brain, including the substantia nigra, basal ganglia, and cerebellum. This accumulation of protein leads to a variety of symptoms including movement disorders, autonomic dysfunction, and cognitive impairment. 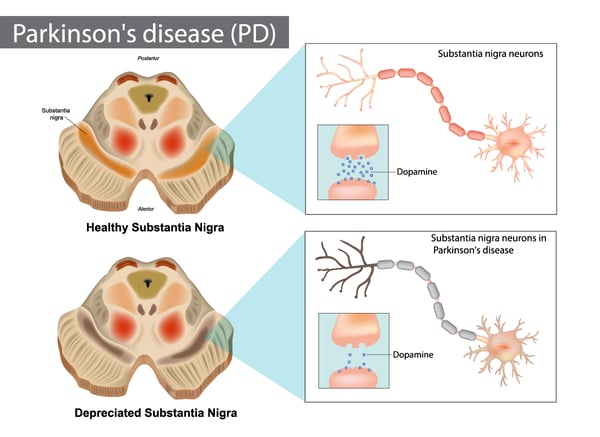 Other disorders, including pure autonomic failure and rapid eye movement (REM) sleep behavior disorder, are also considered synucleinopathies because they are also characterized by the accumulation of alpha-synuclein in certain regions of the brain.
Other disorders, including pure autonomic failure and rapid eye movement (REM) sleep behavior disorder, are also considered synucleinopathies because they are also characterized by the accumulation of alpha-synuclein in certain regions of the brain.
There are currently no disease-modifying therapies for synucleinopathies and no cures for any of these conditions.
Under normal conditions, alpha-synuclein exists in a soluble, unfolded state in the cytoplasm of neurons. However, certain mutations in the alpha-synuclein gene, such as those giving rise to A53T, A30P, and E46K synuclein proteins, or exposure to toxins, such as pesticides, heavy metals, or other environmental factors, may lead to misfolding of the protein into a beta-sheet conformation more prone to aggregation. Traumatic brain injury and other forms of brain damage have also been associated with an increased risk of synucleinopathies. In all cases, aggregation of protein is implicated.
Misfolded alpha-synuclein tends to form oligomers, small clusters of proteins that can aggregate further into insoluble fibrils. These fibrils of protein can form larger aggregates, such as Lewy bodies and Lewy neurites, which are the characteristic features of synucleinopathies. The aggregation of alpha-synuclein is thought to be toxic to neurons, leading to the disruption of cellular processes and ultimately cell death.
The exact mechanisms by which aggregated alpha-synuclein leads to neurodegeneration are not fully understood but are believed to involve the disruption of cellular membranes, interference with degradation pathways for proteins, such as the ubiquitin-proteasome system and autophagy, and activation of inflammatory processes.
The activation of inflammatory processes may lead to the release of pro-inflammatory cytokines and the recruitment of immune cells to affected regions of the brain. This series of events may contribute to the degeneration of neurons and the disruption of neural circuits.
Microplate readers are an invaluable tool to quantify and analyze alpha-synucleins. Not only can they be used to measure the aggregation state of alpha-synucleins but they can also be used to screen for potential inhibitors or drug candidates for the different synucleinopathies. For this purpose, they offer high-throughput capabilities and a wide range of applications.
The levels of alpha-synucleins in biological samples can be determined in several ways, one of which is enzyme-linked immunosorbent assays (ELISA) that are readily amenable to analysis on a microplate. In brief, alpha-synuclein-specific antibodies are immobilized on a microplate and the biological sample incubated with the antibody. The bound alpha-synuclein is detected using a secondary antibody conjugated with an enzyme such as horseradish peroxidase. The enzyme catalyzes a colorimetric or chemiluminescent reaction that can be measured by the microplate reader. This type of assay can be used to monitor changes in alpha-synuclein levels over time.
In the paper “Functional screening of lysosomal storage disorder genes identifies modifiers of alpha-synuclein neurotoxicity”, researchers used a fruit fly model to test 86 conserved homologs of human lysosomal storage disorder genes for interactions with alpha-synuclein-induced neurotoxicity. Variants of the glucocerebrosidase (GBA) gene are common and potent risk factors for Parkinson’s disease. The GBA gene also causes the autosomal recessive lysosomal storage disorder and emerging evidence from genetic studies implicates many other lysosomal storage disorder (LSD) genes in susceptibility to Parkinson’s disease. The authors tested 86 conserved fly homologs of 37 human LSD genes in the aging adult Drosophila brain for potential genetic interactions with neurodegeneration caused by alpha-synuclein. Alpha-synuclein protein levels were detected using an alpha-synuclein human ELISA kit from Invitrogen on microplates where the signal was measured in absorbance at 450 nm on a FLUOstar® OPTIMA microplate reader (discontinued, replaced by the Omega series). The results of the study suggest that partial loss of many LSD genes may enhance alpha-synuclein-mediated Parkinson’s disease and pathogenesis.4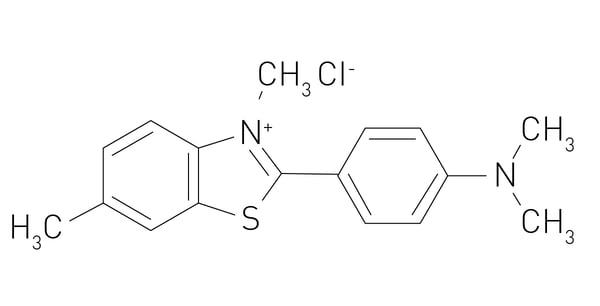 Microplate assays can also be used to perform thioflavin T (ThT) fluorescence assays which are commonly used to measure the aggregation of amyloid proteins. The misfolding and aggregation of alpha-synuclein into amyloid fibrils is a hallmark of synucleinopathies. ThT binds to beta-sheet-rich aggregates such as amyloid fibrils and emits fluorescence on binding. The signal is measured by a fluorescence microplate reader. This technique can be used to monitor the formation of alpha-synuclein aggregates and to assess the effects of inhibitors and other compounds on the aggregation process.
Microplate assays can also be used to perform thioflavin T (ThT) fluorescence assays which are commonly used to measure the aggregation of amyloid proteins. The misfolding and aggregation of alpha-synuclein into amyloid fibrils is a hallmark of synucleinopathies. ThT binds to beta-sheet-rich aggregates such as amyloid fibrils and emits fluorescence on binding. The signal is measured by a fluorescence microplate reader. This technique can be used to monitor the formation of alpha-synuclein aggregates and to assess the effects of inhibitors and other compounds on the aggregation process.
In the paper “A rapid alpha-synuclein seed assay of Parkinson’s disease CSF panel shows high diagnostic accuracy”, a team of researchers described an improved RT-QuIC aggregation assay that can measure the levels of alpha-synuclein in cerebrospinal fluid samples from patients with Parkinson’s disease. Samples were obtained from the MJ Fox Foundation/NINDS BioFIND collection. The improved assay dramatically reduced the time needed to diagnose the disease while maintaining the high-performance standards of previous alpha-synuclein seed assays. The RT-QuIC assays using ThT were carried out on a FLUOstar® Omega microplate reader.5
Two further examples showing the use of ThT assays to look at alpha-synuclein aggregation are described in the papers “Cerebrospinal fluid lipoproteins inhibit alpha-synuclein aggregation by interacting with oligomeric species in seed amplification assays” and “A series of helical alpha-synuclein fibril polymorphs are populated in the presence of lipid vesicles”. In the first study, in vitro aggregation assays were used to help characterize the inhibitory effect of the presence of lipoproteins in cerebrospinal fluid on the detection of alpha-synuclein aggregates. The study reveals apolipoprotein to be the main inhibitory components of CSF and suggests that measurements of apolipoproteins and/or total protein content should be incorporated into data analysis models to eliminate confounding effects of the cerebrospinal fluid environment on alpha-synuclein seed quantification efforts using kinetic parameters.6
In the second study, researchers describe a series of macromolecular assemblies of alpha-synuclein not previously described that form in the presence of lipid vesicles. Alpha-synuclein is naturally located in the neuron in proximity to phospholipids and such assemblies are therefore likely to form. The authors propose that these studies provide the foundation for more-detailed structural analysis and may offer new possibilities to further define disease-relevant versions of the protein that are accessible to pharmacological intervention.7 Studies in both papers involved ThT assays performed on a CLARIOstar® Plus microplate reader to look at alpha-synuclein protein aggregates.
Förster’s Resonance Energy Transfer (FRET), Time-Resolved FRET, and AlphaLISA® are other options for the detection of alpha-synuclein proteins on microplates.
In the paper “Microglial inclusions and neurofilament light chain release follow neuronal alpha-synuclein lesions in long-term brain slice cultures” aggregated alpha-synuclein was measured in brain homogenates and pre-formed fibrils using a HTRF assay developed by Cisbio. The signals from the assays were detected on a PHERAstar® FSX using the TRF laser excitation, Simultaneous Dual Emission at 665 nm / 620 nm and HTRF technology. The study established a quantitative cellular model system for studying alpha-synucleinopathy that contains essential elements of in vivo tissue complexity, and which replicates key aspects of disease. Proof-of-principle was provided for the clinical application of these models in screening antibodies that prevent the spread of alpha-synuclein lesions.8
In the future, research into alpha-synucleins enabled by microplate readers will help advance new applications in high-throughput screening, in vitro aggregation assays, cell-based assays, biomarker identification and the identification of new targets for drug discovery. In drug discovery, screening assays can be performed with small molecules, antibodies, and other therapeutic agents, such as peptide-based inhibitors that decrease the rate of nucleation and fibril elongation, highlighted in the application note ‘Peptidic inhibitors of α-Synuclein that prevent Parkinson-associated fibrilization and cytotoxicity’. The identification of reliable biomarkers for alpha-synuclein pathology is also crucial for the early diagnosis and monitoring of disease progression. Several clinical trials are underway to evaluate the safety and efficacy of antibody treatments that target alpha-synucleins.
The hope is that some much-needed new diagnostic tools and novel treatments can be delivered to patients with these debilitating disorders.
The PHERAstar FSX was specifically conceived for screening campaigns and is your go-to reader for high-performance high-throughput screening.
Both the VANTAstar® and CLARIOstar Plus allow for wavelength flexibility and include Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities. In addition, the Omega series, CLARIOstar Plus and PHERAstar FSX microplate readers come with on-board injectors that can offer the very best options for detection at the time of injection.
BMG LABTECH’s Omega series of readers are a preferred choice for RT-QuIC aggregation assays as they have the robustness to withstand extensive and prolonged shaking.9
Collectively, these multi-mode readers combine high performance with miniaturized assays, short measurement times, and offer considerable savings on materials and other resources.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Neurodegenerative disease ultimately leads to the death of neurons as neuronal functions deteriorate. Find out how microplate readers can be used to study neuronal cell death and its link to neurodegenerative disease.
The disruption of mitochondrial function is linked to neurodegenerative diseases. Find out how microplate readers advance research into mitochondrial dysfunction and neurodegenerative diseases.
The way proteins misfold and aggregate is linked to many neurodegenerative diseases. Find out how microplate readers can help advance research into protein misfolding.
Amyloids are thought to play a crucial role in neurodegeneration. Find out how microplate readers help advance amyloid research.
The tau protein plays a role in many neurological diseases and disorders. Find out about neuronal toxicity induced by tau and how microplate readers can aid tau research.