
PHERAstar FSX
Powerful and most sensitive HTS plate reader
The disruption of mitochondrial function is known to be linked to neurodegenerative diseases. Find out how microplate readers advance research into mitochondrial dysfunction and different neurodegenerative diseases.
 Dr Barry Whyte
Dr Barry Whyte
Mitochondria are organelles enclosed by a double membrane that play a central role in energy production. These powerhouses of the cell generate most of the energy needed to drive essential biological reactions and processes. However, when mitochondria fail to function properly different neurodegenerative diseases can take hold. The disruption of mitochondrial function is a known contributor to neurodegenerative diseases like Parkinson’s and Alzheimer’s disease, although the precise details remain to be worked out.
In this blog, we look at the relationship between mitochondrial dysfunction and neurodegenerative disease. We also discuss how microplate readers can be used in neuroscience research to reveal the impact mitochondrial dysfunction has on health.
Mitochondria play a fundamental role in cell metabolism: they generate most of the cell’s supply of adenosine triphosphate (ATP), the energy currency of the cell. They carry out aerobic respiration, a process that involves the breakdown of glucose and other fuel molecules to produce ATP through a series of interconnected reactions, including the citric acid cycle and the mitochondrial electron transport chain.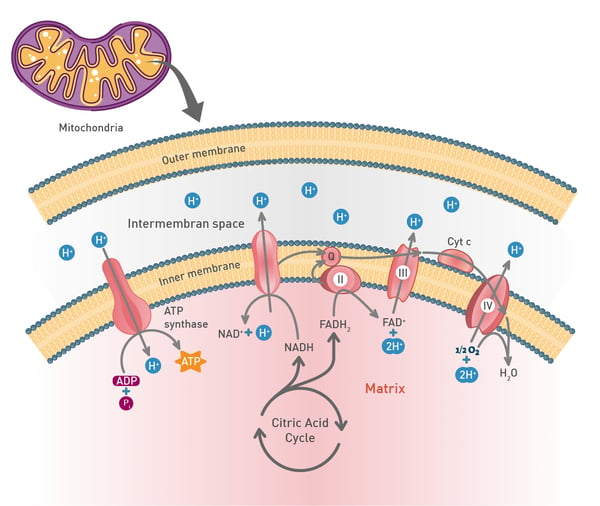 Many factors can contribute to mitochondrial dysfunction including genetic mutations, aging, environmental factors, nutritional deficiencies, and metabolic disorders. The consequences for the cell can be dramatic mainly due to a reduction in the amount of ATP produced and an increase in the levels of reactive oxygen species. Increased levels of reactive oxygen species can damage the molecular fabric of the cell including lipids, proteins, and DNA.1 Excessive production of reactive oxygen species can lead to oxidative stress which is associated with inflammation and neurodegenerative disease. In addition, insufficient levels of ATP directly affect the function of tissues and essential organs like the brain. Other effects include impaired cellular respiration, altered cell signaling, apoptosis, and impaired calcium homeostasis.
Many factors can contribute to mitochondrial dysfunction including genetic mutations, aging, environmental factors, nutritional deficiencies, and metabolic disorders. The consequences for the cell can be dramatic mainly due to a reduction in the amount of ATP produced and an increase in the levels of reactive oxygen species. Increased levels of reactive oxygen species can damage the molecular fabric of the cell including lipids, proteins, and DNA.1 Excessive production of reactive oxygen species can lead to oxidative stress which is associated with inflammation and neurodegenerative disease. In addition, insufficient levels of ATP directly affect the function of tissues and essential organs like the brain. Other effects include impaired cellular respiration, altered cell signaling, apoptosis, and impaired calcium homeostasis.
Several lines of evidence suggest the involvement of mitochondrial dysfunction in Alzheimer’s disease. In addition to less ATP production and higher levels of reactive oxygen species, mitochondria in the brains of individuals with Alzheimer’s disease show mitochondrial abnormalities that include altered shape, decreased density, and impaired respiration.
Evidence also points to multiple genes being linked to mitochondrial dysfunction in Alzheimer’s disease. They include mutations in the genes encoding for amyloid precursor protein as well as presenilin 1 and presenilin 2, two transmembrane proteins that constitute the catalytic subunit of gamma-secretase, the so-called “proteosome of the membrane”. In addition, mutations in mitochondrial DNA have been associated with an increased risk of the disease.
Reduced energy production may contribute to neuronal dysfunction and synaptic loss.
In this context, studies using positron emission tomography have indicated decreased use of glucose and impaired oxidative metabolism in the brains of patients with Alzheimer’s disease.2
Altered mitochondrial dynamics, including excessive fission and fusion of mitochondria, also contribute to mitochondrial dysfunction in Alzheimer’s disease. This can impair processes like mitochondrial transport and the clearance of proteins leading to bioenergetic deficits and neuronal dysfunction.
In Parkinson’s disease, a strong link between mitochondrial dysfunction and disease-associated genes has been documented. Loss of function of the Parkin gene (PARK2) leads to impaired mitophagy (destruction of mitochondria by the cell’s homeostatic machinery) which produces a buildup of damaged mitochondria. Mutations in the PINK1 gene (also known as PARK6) are associated with early onset Parkinson’s disease. When mitochondria become depolarized or damaged, the PINK1-encoded protein, which is a ubiquitin kinase, accumulates on the outer membrane. PINK1 acts as a sensor of mitochondrial health and works in coordination with Parkin to maintain mitochondrial quality and function.3
Mutations in the DJ-1 gene (also known as PARK7) are known to be linked with early onset Parkinson’s disease. DJ-1, a protein of the peptidase C56 family, protects against mitochondrial oxidative stress and maintains mitochondrial integrity (Fig. 2). DJ-1 serves as a promising biomarker and therapeutic target for Parkinson’s disease as well as a wider range of other neurodegenerative diseases.4
 Mutations in the LRRK2 gene (also known as PARK8) are the most common genetic cause of familial and sporadic Parkinson’s disease. Studies suggest that LRRK2 (leucine-rich repeat kinase 2) may influence mitochondrial morphology, respiration, and calcium homeostasis. Other genes, including SNCA (alpha-synuclein), ATP13A2, and FBXO7, are also linked to mitochondrial dysfunction and Parkinson’s disease.
Mutations in the LRRK2 gene (also known as PARK8) are the most common genetic cause of familial and sporadic Parkinson’s disease. Studies suggest that LRRK2 (leucine-rich repeat kinase 2) may influence mitochondrial morphology, respiration, and calcium homeostasis. Other genes, including SNCA (alpha-synuclein), ATP13A2, and FBXO7, are also linked to mitochondrial dysfunction and Parkinson’s disease.
Beyond genetics, other lines of evidence suggest a link between mitochondrial dysfunction and Parkinson’s disease. Lower ATP production contributes to the degeneration of dopaminergic neurons in the substantia nigra. An increase in reactive oxygen species can also contribute to the degeneration of dopaminergic neurons. Impaired mitophagy, calcium dysregulation, mutations in mitochondrial DNA, and impaired mitochondrial dynamics are also implicated.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that mainly impacts motor neurons. Evidence has been steadily accumulating for a link between mitochondrial abnormalities and ALS. Mitochondrial DNA mutations have been observed in a subset of ALS patients. Alterations to the morphology of mitochondria, reduced mitochondrial membrane potential, and increased mitochondrial fragmentation have also been reported in model systems of ALS and in patient-derived cells.
Microplate readers offer many capabilities that can be applied to the study of mitochondrial dysfunction. They can be used for example to investigate mitochondrial respiration, metabolic activity, oxygen consumption and ATP production. They can also be used to look at the opening of the mitochondrial permeability transition pore (mPTP), which is associated with cell death and mitochondrial damage. Microplate readers are also ideally suited for high-throughput screening assays. Applications include screening for therapeutics that aim to improve mitochondrial function. Here we take a closer look at some of these uses.
Microplate readers can assess mitochondrial respiration by measuring oxygen consumption rates. One commonly used method involves using oxygen-sensitive fluorescent dyes such as the MitoXpress and MitoXpress-Intra assays from Agilent. These methods can be used to determine respiration rates for metabolic characterization and to evaluate mitochondrial dysfunction in a high-throughput format. More information on how MitoXpress-Extra allows microplate-based analysis of mitochondrial function through the quantitation of oxygen consumption is available in the MitoXpress-Xtra blog from BMG LABTECH.
Microplate readers can accommodate several assays to evaluate metabolic activity. The Vybrant® MTT assay is often employed to assess cell viability and metabolic function. Cells are incubated with the dye, which is converted to a formazan product by metabolically active cells. After solubilizing the formazan, the microplate reader measures the absorbance at a specific wavelength, which correlates with the metabolic activity. Other assays like CellTiter-Glo® or resazurin-based assays also provide measurements of metabolic activity based on the detection of ATP levels or cell viability, respectively (Fig. 3).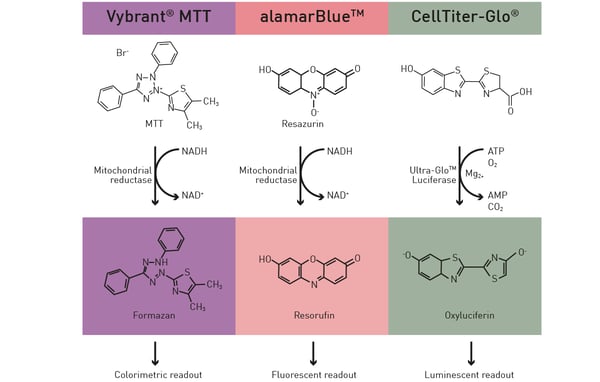 You can read more about some of these viability assays in the application note “Viability assays: a comparison of luminescence-, fluorescence-, and absorbance-based assays to determine viable cell counts”. The application note describes how some of the commonly used viability assays can be performed on the VANTAstar® microplate reader.
You can read more about some of these viability assays in the application note “Viability assays: a comparison of luminescence-, fluorescence-, and absorbance-based assays to determine viable cell counts”. The application note describes how some of the commonly used viability assays can be performed on the VANTAstar® microplate reader.
Microplate readers are an effective way to measure oxygen consumption rates. For example, the application note “Measuring changes in cellular metabolism by monitoring extracellular acidification and oxygen consumption in real-time” describes how an open flow respirometry method for the measurement of mitochondrial respiration rates at dynamic steady-state oxygen concentrations was translated to a 96-well microplate format. The assays were performed on a CLARIOstar® Plus equipped with an Atmospheric Control Unit (ACU). As an example of the capabilities of this test for compound screening, a dose-response curve was prepared to the known mitochondrial inhibitor antimycin A (Fig. 4). The CLARIOstar® Plus with ACU provided a key element in the open-flow respirometry system.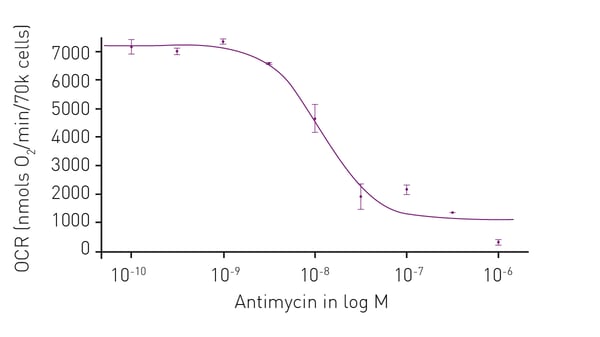 As mentioned earlier, the levels of ATP are a useful diagnostic for mitochondrial health and therefore a window to look at the impact on neurodegenerative disease. In the application note Promega ENLITEN® kit performed on a BMG LABTECH microplate reader, ATP levels were measured to limits of detection beyond the linearity of the Promega ENLITEN kit. Detection was by luminescence plate reader at a high sensitivity suitable for the measurement of ATP concentrations. The performance of the ENLITEN kit was linear over a range of 10 fmol/well to 0.195 fmol/well (Fig. 5). Monitoring ATP levels with the ENLITEN kit is not only a barometer for neurodegenerative health, it also provides a means to look at the different effects of therapeutics on different targets.
As mentioned earlier, the levels of ATP are a useful diagnostic for mitochondrial health and therefore a window to look at the impact on neurodegenerative disease. In the application note Promega ENLITEN® kit performed on a BMG LABTECH microplate reader, ATP levels were measured to limits of detection beyond the linearity of the Promega ENLITEN kit. Detection was by luminescence plate reader at a high sensitivity suitable for the measurement of ATP concentrations. The performance of the ENLITEN kit was linear over a range of 10 fmol/well to 0.195 fmol/well (Fig. 5). Monitoring ATP levels with the ENLITEN kit is not only a barometer for neurodegenerative health, it also provides a means to look at the different effects of therapeutics on different targets.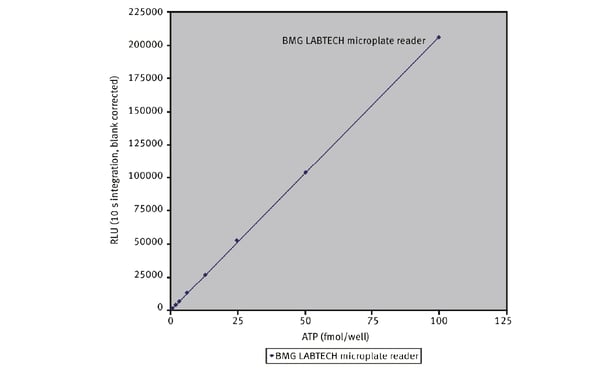
The mitochondrial permeability transition pore is associated with cell death and mitochondrial damage which in turn is linked to neurodegenerative disease. Microplate readers can be used to perform real-time measurements of fluorescence intensity that allow monitoring of the opening over time of the mitochondrial permeability transition pore. Cells or mitochondria are typically loaded with the fluorescent probe and placed in the microplate wells. The microplate reader then measures the fluorescence at regular intervals providing a time course for opening of the channel. The kinetics and dynamics of the process can therefore be studied along with the impact of potential inhibitors or activators of the channel. One of the advantages of using a microplate reader for this type of assay is that the analysis can be performed at high throughput.
The application note “Calcium retention capacity assay evaluates inhibition of mitochondrial permeability transition pore” describes the use of the dye Calcium Green™ 5N to determine the calcium retention capacity (CRC) of mitochondria. Calcium Green™ 5N exhibits an increase in fluorescence emission intensity upon binding to calcium. The CRC assay is a high-throughput assay for the screening of mitochondrial permeability transition pore (MPTP) inhibitors. It can also be adopted for use with live permeabilized cells.
Microplate readers are also valuable tools for high-throughput screening applications related to drug discovery and development for mitochondrial dysfunction. They can be used for high-throughput assays to look for potential therapeutics that target mitochondria with the aim of improving mitochondrial function. For this purpose, assays can be designed to target specific steps of the drug development process including assay selection, construction of compound libraries, sample preparation, hit validation and other studies. The combination of automation, high-throughput capabilities, and diverse readout options make microplate readers extremely useful tools in this process.
Researchers are actively pursuing different ways to target mitochondrial dysfunction.5 Some of these approaches for neurodegenerative diseases include exploring antioxidant therapies, targeting mitochondrial dynamics, enhancing the biogenesis of mitochondria, and improving energy production.
Antioxidant therapies aim to counteract the harmful effects of reactive oxygen species. Targeting mitochondrial dynamics, for example by enhancing mitochondrial fusion, can promote the exchange of mitochondrial components and rescue dysfunctional mitochondria. Inhibiting excessive fission of mitochondria can prevent mitochondrial fragmentation and dysfunction. Researchers are also exploring pharmacological agents, lifestyle interventions, and other approaches to enhance mitochondrial biogenesis. In cases where energy production is impaired due to neurodegenerative diseases, strategies that improve energy production in mitochondria are being pursued. This includes interventions to enhance oxidative phosphorylation, increase substrate availability for mitochondrial respiration, and ways to improve the function of the mitochondrial electron transport chain.
While progress has been made in understanding the relationship between mitochondrial dysfunction and neurodegenerative disease, challenges remain on several fronts for drug discovery and development. More effective drug delivery systems is one example of what is needed. New approaches that can selectively target mitochondria and deliver therapeutic agents in a controlled manner are therefore high on the priority list. Early detection and intervention strategies will be crucial for success.
Interventions that target mitochondria to curtail neurodegenerative diseases must not only be effective but also suitable for long-term use with little or no side effects. In practice, it may be necessary to combine several different approaches to restore mitochondrial function. Microplate readers offer researchers a flexible, powerful tool to approach these challenges using new and existing assays.
The PHERAstar FSX was specifically conceived for screening campaigns and is your go-to reader for high-performance high-throughput screening.
Both the VANTAstar® and CLARIOstar Plus allow for wavelength flexibility and include Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities. In addition, the Omega series, CLARIOstar Plus and PHERAstar FSX microplate readers come with on-board injectors that can offer the very best options for detection at the time of injection.
BMG LABTECH’s Omega series of readers are a preferred choice for RT-QuIC aggregation assays as they have the robustness to withstand extensive and prolonged shaking.6
Collectively, these multi-mode readers combine high performance with miniaturized assays, short measurement times, and offer considerable savings on materials and other resources.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Neurodegenerative disease ultimately leads to the death of neurons as neuronal functions deteriorate. Find out how microplate readers can be used to study neuronal cell death and its link to neurodegenerative disease.
The way proteins misfold and aggregate is linked to many neurodegenerative diseases. Find out how microplate readers can help advance research into protein misfolding.
Alpha-synuclein is a key protein involved in neurodegenerative diseases like Parkinson’s. Find out how microplate readers can help advance alpha-synuclein research.
Amyloids are thought to play a crucial role in neurodegeneration. Find out how microplate readers help advance amyloid research.
The tau protein plays a role in many neurological diseases and disorders. Find out about neuronal toxicity induced by tau and how microplate readers can aid tau research.