
NEPHELOstar Plus
Microplate nephelometer for light-scattering and turbidity measurements
Amyloids are thought to play a crucial role in neurodegeneration. Find out how microplate readers help advance research into amyloid aggregation and neurodegenerative disease.
 Dr Barry Whyte
Dr Barry Whyte
Early research into Alzheimer’s disease indicated the importance of amyloid-beta as a crucial component of senile plaques in the brain. In parallel, the tau protein was revealed as the main component of neurofibrillary tangles in patients with the disease.
Today, the exact causes of Alzheimer’s disease remain to be established. The onset and development of the disease may involve the intersection of multiple factors including interactions between amyloid-beta, tau and molecular responses like inflammation. However, the precise origins of Alzheimer’s remain the subject of intense research.
According to the World Health Organization, more than 55 million people worldwide have dementia. Alzheimer’s disease, its most common form, may contribute to 60-70% of cases.
Historically, it is the “amyloid hypothesis”, which maintains that amyloid-beta is the major driving factor in the development of Alzheimer’s, that has served as the predominant framework for research into the disease. The “amyloid hypothesis” has been a major focus for the development of potential treatments for around 30 years.
In this blog, we look at the crucial role of amyloid in Alzheimer’s disease and discuss some of the recent progress in research and the development of therapeutics. We also highlight how microplate readers can be used in neuroscience to accelerate research into amyloid.
Amyloids are insoluble, protein aggregates that comprise highly ordered fibrils with a beta-sheet structure (fig. 1). They can accumulate in different tissues and organs and are linked to several diseases and conditions including Alzheimer’s, Parkinson’s and prion diseases. The formation of amyloid fibrils involves the conversion of normally soluble proteins into highly ordered, aggregated structures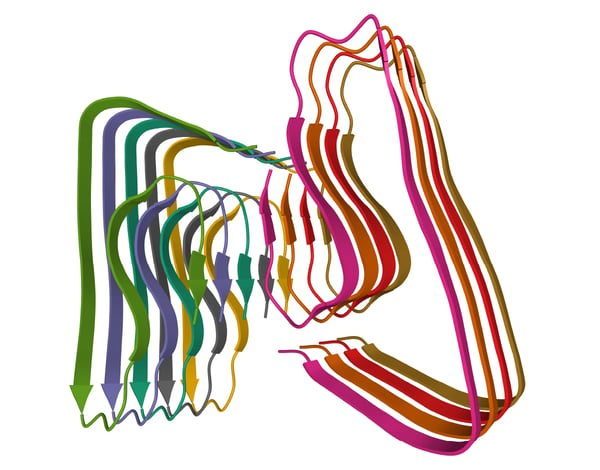 The accumulation of amyloid fibrils is thought to disrupt cellular processes in a way that leads to toxicity and cell death. In some cases, the accumulation of amyloid is closely linked to inflammation and activation of the immune system, which extends tissue damage even further.
The accumulation of amyloid fibrils is thought to disrupt cellular processes in a way that leads to toxicity and cell death. In some cases, the accumulation of amyloid is closely linked to inflammation and activation of the immune system, which extends tissue damage even further.
In Alzheimer’s disease, amyloid-beta peptides accumulate in the brain and form plaques. These plaques are believed to contribute to cognitive impairment and dementia. In Parkinson’s disease, alpha-synuclein forms amyloid fibrils that accumulate in the brain, and which contribute to the death of dopaminergic neurons. These molecular events result in movement disorders such as tremors and rigidity. The precise mechanism by which amyloids contribute to disease and other neurodegenerative conditions are not yet known. Research is focused on understanding these processes to develop new treatments and help prevent the onset of amyloid-related diseases.
The cross-beta sheet structure of amyloids is a highly ordered arrangement of beta strands stacked perpendicular to the fibril axis. The beta strands are held together by hydrogen bonds that run parallel to the fibril axis. The cross-beta sheet structure is very stable, extremely rigid and resistant to degradation by proteases and other enzymes. The fibrils have a diameter of around 10 nanometers and can vary in length from a few nanometers to several microns. The individual protein subunits within the fibrils are organized in a highly repetitive fashion. While the cross-beta sheet structure is a defining characteristic of all amyloid fibrils, the precise structure of the fibrils can vary depending on the specific proteins involved.
Protein misfolding is a critical step in the formation of amyloids. Typically, proteins fold into unique three-dimensional structures that are needed for them to function correctly. However, under certain conditions proteins can misfold leading to the formation of partially folded or unfolded intermediates that are prone to aggregation. Misfolded proteins can interact with each other and form small aggregates or oligomers. These smaller aggregates may grow into larger fibrils and eventually form amyloid deposits.
What gives rise to protein misfolding? Several factors may contribute including genetic mutations, environmental factors, and even age-related changes in the body. In many cases, misfolded proteins are cleared from the body by mechanisms such as proteosomal degradation or autophagy. However, if the rate of misfolding exceeds the rate of clearance, misfolded proteins will accumulate and may form into amyloid deposits.
Amyloids are thought to contribute in several ways to neurodegenerative diseases like Alzheimer’s. First amyloid fibrils may interact with cellular receptors in a way that disrupts cellular signaling pathways. The formation of amyloid fibrils can also increase the production of reactive oxygen species, which can cause oxidative damage to cells and tissues. As mentioned earlier, the accumulation of amyloids is also linked to inflammation and may activate the immune system. Some studies implicate an inflammatory cascade in the early steps of Alzheimer’s disease, which may also involve other factors like tau phosphorylation, mitochondrial damage, and other peripheral components.1
Another way amyloid fibrils can contribute to neurodegeneration is by interfering with cellular transport processes. This type of hindrance can result in the accumulation of toxic substances in cells.
The ability to understand the mechanisms by which amyloids contribute to neurodegenerative diseases is essential if new treatments and therapies are to be introduced.
Much effort has gone into trying to target amyloids with drugs that prevent or slow the progression of disease but only a few have received regulatory approval. The R&D approach has focused on several fronts. This includes looking for therapeutic agents that can block beta sheet formation, prevent the synthesis of fibrils, dissolve amyloid-beta aggregates into non-toxic species, destabilize amyloid-beta oligomers or accelerate the conversion of amyloid-beta oligomers to amyloid-beta aggregates.2,3
Some studies have questioned whether the formation of amyloid plaques is always a negative.3,4
Functional amyloids have been reported to have antioxidant and antibacterial effects.
Chaperone-dependent amyloid assembly has also been reported to protect cells from prion toxicity. 5 Therefore amyloids may also play a beneficial role in cells and organisms and not just contribute to disease and other conditions.
Several acetylcholinesterase inhibitors have been approved for the treatment of Alzheimer’s disease. They include Donepezil, rivastigmine and galantamine. While they do not directly target amyloid fibrils, they can improve cognitive function in some patients.
Memantine, an N-methyl-D-aspartate receptor antagonist, is approved for the treatment of moderate to severe Alzheimer’s disease. It works by reducing the damage due to excess glutamate, a neurotransmitter that is released in large amounts in Alzheimer’s patients. Another option could be the development of inhibitors of aggregation as highlighted in the application note ‘Peptidic inhibitors of α-Synuclein that prevent Parkinson-associated fibrilization and cytotoxicity’.
More recently, several drugs have been developed that target amyloid-beta directly. Examples include the monoclonal antibody aducanumab, recently approved by the FDA, and donanemab which is currently in later-stage clinical trials. Prior to these two drugs, almost two dozen clinical trials on drugs seeking to treat Alzheimer’s by reducing amyloid plaques had failed since 2003. 6 New treatments and approaches are much needed. While there are signs of progress, debate continues about the merits of the amyloid hypothesis in the research and medical communities. This has been fueled by differences in opinions among experts. Much of the work is ongoing but the hope is that progress will spawn more effective therapies and treatments.
You can learn more about Alzheimer’s disease target identification and validation on the PHERAstar FSX in the following video:

Microplate readers are often used in amyloid research to measure the formation or accumulation of amyloid fibrils in vitro and are useful tools to monitor changes in absorbance or fluorescence over time. They offer high-throughput capabilities and a range of applications.
Fluorescence-based assays can readily be used to detect amyloid fibrils in vitro. Thioflavin T (ThT) is a dye that binds to amyloid fibrils and fluoresces on binding. ThT is a benzothiazole salt that shows increased fluorescence when bound to beta sheet-rich structures such as amyloid fibrils of amyloid-beta. The formation of amyloid fibrils can be quantified by monitoring the fluorescent intensity of ThT-bound samples over time. A ThT assay is a highly sensitive assay to look at large numbers of samples in high-throughput assays suitable for drug discovery.
In the application note Following Abeta fibrillization/aggregation in real-time using a FLUOstar® Omega microplate reader an in vitro cell-free assay using ThT was used to detect amyloid seeding activity in brain samples (fig. 2).  The assay allows for a statistically validated, quantitative comparison of amyloid seeding activity in different samples. The FRANK-Assay, which stands for Fibrillization of Recombinant Amyloid-beta Nucleation Kinetics, was used to determine the amount of amyloid aggregation seeds in brain tissue homogenates. The assay was run over 2-3 days on a FLUOstar® Omega microplate reader.
The assay allows for a statistically validated, quantitative comparison of amyloid seeding activity in different samples. The FRANK-Assay, which stands for Fibrillization of Recombinant Amyloid-beta Nucleation Kinetics, was used to determine the amount of amyloid aggregation seeds in brain tissue homogenates. The assay was run over 2-3 days on a FLUOstar® Omega microplate reader.
All signal curves showed a clear increase in fluorescence (fig. 3) that represents the incorporation of ThT into newly formed amyloid-beta fibrils. After some time, a plateau is reached that represents an endpoint of amyloid formation and the cessation of the ThT incorporation process.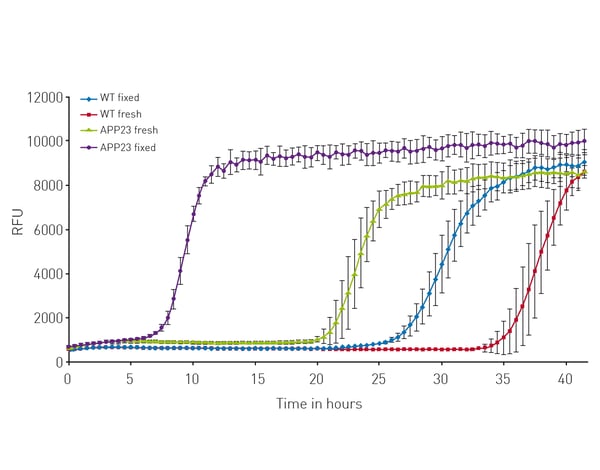 The lag times derived from the signal curves are a useful measure of the fibrilization process. Moreover, the assay delivers significant time savings compared with typical in vivo assays (a 4-6 month in vivo assay could be reduced to a 2–3-day in vitro assay).
The lag times derived from the signal curves are a useful measure of the fibrilization process. Moreover, the assay delivers significant time savings compared with typical in vivo assays (a 4-6 month in vivo assay could be reduced to a 2–3-day in vitro assay).
In the application note Novel aggregation-specific fluorogen monitors prefibrillar protein aggregation by fluorescence polarization an alternative fluorogen to ThT was used to look at prefibrillar aggregation using fluorescence polarization (fig. 4). 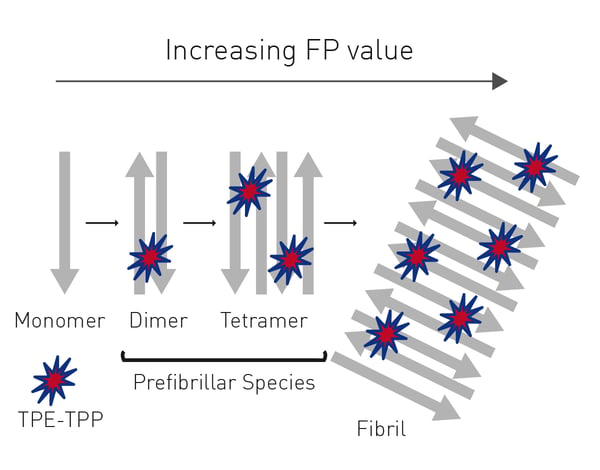 The broad-spectrum fluorescent probe bis[triphenylphosphonium] tetraphenylethene (TPE-TPP) has emission characteristics that are ideally suited to the monitoring of prefibrillar aggregation.
The broad-spectrum fluorescent probe bis[triphenylphosphonium] tetraphenylethene (TPE-TPP) has emission characteristics that are ideally suited to the monitoring of prefibrillar aggregation.
One advantage of the TPE-TPP probe is that it is better suited to detect the presence of prefibrillar species formed during the early stages of protein aggregation.
The use of TPE-TPP is well suited to study amyloid aggregation of proteins. The fluorescence polarization signal is dependent on the increasing mass of the probe/protein complex and less dependent on the fluctuation of fluorescence intensity caused by the inhomogeneity of the solution.
The fluorescence polarization results for the aggregation of bovine insulin are shown in Fig. 5. The use of the probe clearly shows the progression from monomeric insulin to oligomers and then to insulin amyloid fibrils by stepwise increases in fluorescence polarization. The assays were run on a CLARIOstar® microplate reader.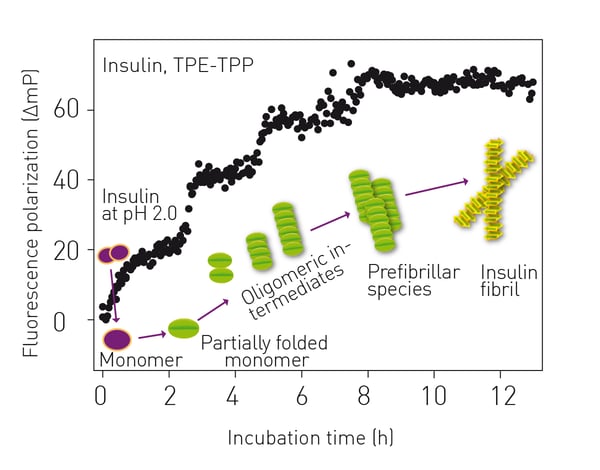 In the paper HSF1 physically neutralizes amyloid oligomers to empower overgrowth and bestow neuroprotection researchers used a CLARIOstar® microplate reader for in vitro amyloid seeding assays using the dye ThT.7 The researchers also used turbidity measurements on a NEPHELOstar® Plus to look at how Heat shock factor 1 physically neutralizes soluble amyloid oligomers. Amyloid-beta1-42 peptides were mixed with recombinant HSF1 protein at different molar ratios together with mouse brain lysates in a 96-well microplate. Samples were incubated with shaking for 48 hours. A simple addition of recombinant HSF1 to the brain lysates blocked the increase in turbidity and the formation of prefibrillar amyloid oligomers completely. The turbidity measurements likely detected the formation of high molecular weight complexes between amyloid oligomers and cellular proteins such as amyloid oligomer-heat shock protein 60/chaperonin complexes. Laser-based nephelometry therefore offers opportunities to study aggregation of amyloid proteins for screening purposes.
In the paper HSF1 physically neutralizes amyloid oligomers to empower overgrowth and bestow neuroprotection researchers used a CLARIOstar® microplate reader for in vitro amyloid seeding assays using the dye ThT.7 The researchers also used turbidity measurements on a NEPHELOstar® Plus to look at how Heat shock factor 1 physically neutralizes soluble amyloid oligomers. Amyloid-beta1-42 peptides were mixed with recombinant HSF1 protein at different molar ratios together with mouse brain lysates in a 96-well microplate. Samples were incubated with shaking for 48 hours. A simple addition of recombinant HSF1 to the brain lysates blocked the increase in turbidity and the formation of prefibrillar amyloid oligomers completely. The turbidity measurements likely detected the formation of high molecular weight complexes between amyloid oligomers and cellular proteins such as amyloid oligomer-heat shock protein 60/chaperonin complexes. Laser-based nephelometry therefore offers opportunities to study aggregation of amyloid proteins for screening purposes.
Microplate readers can also be used to screen for compounds that inhibit the formation or accumulation of amyloid fibrils or to look at the promotion or inhibition of protein misfolding. These types of assays can also involve fluorescent dyes like ThT.
Amyloid research is evolving rapidly. Advances in imaging and spectroscopic techniques are allowing researchers to visualize and track the formation and spread of amyloids in real time, which provides new insights into the progress and spread of the disease.
Diagnostics continue to be an important area of research since there is a large need for the early detection of disease. Critically, biomarkers that detect the presence of amyloid fibrils or related proteins in blood or cerebrospinal fluid are being developed and tested for their ability to identify individuals at risk of developing amyloid-related disorders before the onset of clinical symptoms.
What is the preferred BMG LABTECH microplate reader for specific needs and applications related to amyloid research? BMG LABTECH offers a range of detection devices for sensitive absorbance and fluorescence measurements.
The PHERAstar® FSX was specifically conceived for screening campaigns and is your go-to reader for high-performance high-throughput screening.
Both the VANTAstar® and CLARIOstar® Plus allow for emission scanning and include Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities. In addition, the Omega series, CLARIOstar Plus and PHERAstar FSX microplate readers come with on-board injectors that can offer the very best options for detection at the time of injection.
BMG LABTECH’s Omega series of readers are a preferred choice for RT-QuIC aggregation assays as they have the robustness to withstand extensive and prolonged shaking.7
The NEPHELOstar Plus is the world’s only laser-based microplate nephelometer and offers a high-throughput option to look at amyloid-beta and other types of protein aggregation in screening assays.
Collectively, these multi-mode readers combine high performance with miniaturized assays, short measurement times, and offer considerable savings on materials and other resources.
Microplate nephelometer for light-scattering and turbidity measurements
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Neurodegenerative disease ultimately leads to the death of neurons as neuronal functions deteriorate. Find out how microplate readers can be used to study neuronal cell death and its link to neurodegenerative disease.
The disruption of mitochondrial function is linked to neurodegenerative diseases. Find out how microplate readers advance research into mitochondrial dysfunction and neurodegenerative diseases.
The way proteins misfold and aggregate is linked to many neurodegenerative diseases. Find out how microplate readers can help advance research into protein misfolding.
Alpha-synuclein is a key protein involved in neurodegenerative diseases like Parkinson’s. Find out how microplate readers can help advance alpha-synuclein research.
The tau protein plays a role in many neurological diseases and disorders. Find out about neuronal toxicity induced by tau and how microplate readers can aid tau research.