
SPECTROstar Nano
Absorbance plate reader with cuvette port
Endotoxins are a component of the outer membrane of Gram-negative bacteria. Their detection is vital in the pharmaceutical and medical industry for product quality and safety.
 Dr Tobias Pusterla
Dr Tobias Pusterla
Endotoxins are the main component of the outer membrane of the cell wall of Gram-negative bacteria. Typically, the term endotoxin is used synonymously with lipopolysaccharide (LPS), despite the fact that a few endotoxins are not LPS. The main function of endotoxins in bacteria is structural and protective.
Gram-negative bacteria are characterised by two membranes: the inner membrane surrounds the cytoplasma whereas the outer membrane separates the bacterial cell wall from the external environment. Thus, the outer membrane serves as the first line of defence against environmental threats. In most cases, the outer membrane is not a common phospholipid bilayer but an asymmetric bilayer comprising LPS in the outer layer and phospholipids in the inner one (fig.1).
Lipid A is the toxic component of endotoxins. It is a phosphorylated N-acetylglucosamine disaccharide containing a hydrophobic part (aliphatic chains of fatty acids) that anchors the endotoxin into the bacterial membrane. The rest of the endotoxin projects from the cell surface (fig.1). Although its structure is extremely conserved, it can undergo modifications in response to varying environmental conditions.1
The O-antigen is attached to the core polysaccharide and is the outermost part of the molecule. Although not toxic, it is the main immunogenic portion of endotoxins and consequently, it is a recognition target for antibodies and a major antigenic determinant. It is a repetitive glycan polymer made up of 3 to 5 sugars. It is the most diverse component of LPS: composition and length vary among species and even strains of bacteria.
Endotoxins are the main component of the outer membrane of Gram-negative bacteria and of vital importance to their survival. Endotoxins contribute to the structural integrity of bacteria and act as a protective amphipathic barrier, shielding bacteria from chemical attacks. Endotoxins establish a barrier that is permeable only to hydrophilic molecules with low molecular weight, making Gram-negative bacteria resistant to many antimicrobial compounds.3
The reduced permeability to large hydrophilic molecules mainly results from the hydrophobic nature of Lipid A. The hydrophilic nature of the core oligosaccharide and O-antigen additionally make endotoxins impermeable to hydrophobic compounds.
In hosts, LPS protects bacteria from killing by phagocytes or serum components. Of notice, variations in the endotoxin structure establish different antigenic strains, increasing their chance of circumventing immunological responses that were previously developed against a specific strain of bacteria, allowing resistance to evolve.
As endotoxins are exposed on the surface of bacteria, the innate immune system has evolved to recognise them as a threat and to react accordingly to their presence. Endotoxins are pyrogens, provoking a strong innate immune response. When Gram-negative bacteria are killed by the immune system, fragments of their membrane containing endotoxins are released in the blood stream and may cause fever and diarrhoea. The presence of endotoxins in the blood (endotoxemia) typically leads to hypotension, respiratory failure and reduced oxygen delivery.4 Strong endotoxemia can lead to sepsis and eventually death.
Being the most conserved portion of LPS, the immune system has evolved to respond predominantly to Lipid A. The immune reaction is entirely innate and mediated by Toll-Like Receptor 4 (TLR4) in complex with MD2 (fig.4).5
TLR4 stimulates the secretion of pro-inflammatory cytokines and nitric oxide from macrophages and endothelial cells. In addition, endotoxins stimulate B-cell differentiation, proliferation and immunoglobulin secretion (mainly IgG and IgM). In macrophages and monocytes, endotoxins trigger the production of inflammatory cytokines (such as interleukin-1, 6 and 8, TNF and platelet-activating factor) and the consequent release of prostaglandins and leukotrienes.6 Moreover, the complement and coagulation cascades are activated inducing inflammation, vasodilation, chemotaxis of neutrophils, coagulation, bleeding and shock.
The O antigen is the immunogenic part of endotoxins, leading to antibody production from the host and contributing to evasion of phagocytosis. The involvement of the O antigen is confirmed by the fact that changes in its polysaccharide sequence significantly affect virulence. However, the mechanism underlying polysaccharide-driven virulence is not fully understood yet.
There are several methods to test for the presence of endotoxins. In vitro endotoxin testing methods include LAL assay and ELISA. Both can be run on microplate readers, significantly increasing throughput and efficiency.
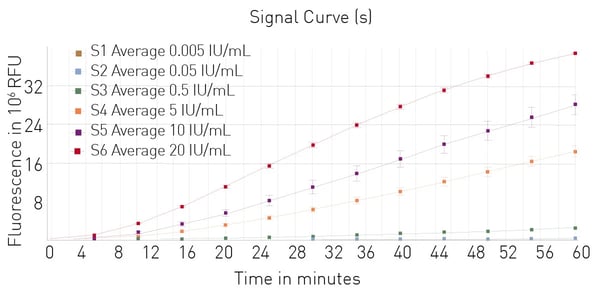
The Limulus Amebocyte Lysate (LAL) assay is a common bacterial endotoxin testing method (BET test). The LAL test is based on an extract of isolated amebocytes from the blood of the horseshoe crab. LAL tests are either chromogenic or turbidimetric (See AN409 "Detection of bacterial endotoxins using the PYROSTAR™ ES-F/Plate LAL Assay" or AN412 "Colorimetric and turbidimetric analysis of endotoxins using the absorbance detection mode"). A LAL substitute test based on recombinant proteins and a fluorescent substrate is also available (fig. 5). Find more information on our blog post: “The LAL assay: a living fossil exploited to detect bacterial contamination.”
 Endotoxins can also be assayed by ELISA which can detect either directly endotoxins or anti-endotoxin antibodies. However, the amphipathic nature of endotoxins negatively affects binding on ELISA plates and results in variable conformations of epitope binding sites. The result is generally low sensitivity and poor reproducibility.
Endotoxins can also be assayed by ELISA which can detect either directly endotoxins or anti-endotoxin antibodies. However, the amphipathic nature of endotoxins negatively affects binding on ELISA plates and results in variable conformations of epitope binding sites. The result is generally low sensitivity and poor reproducibility.
All these endotoxin assays can be measured on a microplate reader. These approaches generally require an absorbance microplate reader to detect either a chromogenic reaction (LAL and most typically ELISA), or the changes in turbidity. For assays based on recombinant proteins and a fluorescent substrate, a fluorescence microplate reader is necessary.
BMG LABTECH plate readers that can be used to quantify endotoxins include the SPECTROstar Nano, Omega series, VANTAstar, CLARIOstar Plus and PHERAstar FSX.
Absorbance plate reader with cuvette port
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Learn about the bacterial endotoxin test (BET test) and its role in ensuring the safety of pharmaceuticals, biologics and medical devices.
The monocyte activation test is a cell-based assay used to detect pyrogens. Learn more about the monocyte activation test and how microplate readers can support these crucial tests for quality control and bioanalysis.
The recombinant factor C (rFC) test is used to detect bacterial endotoxins that can cause fever and adverse events when introduced into the body. Learn about the rFC test and the advantages it offers for endotoxin detection in the life sciences.
Pyrogen tests are vital to ensure the safety of different health interventions including pharmaceuticals, medical devices and an array of biological products. This blog looks at the different types of pyrogens as well as some of the widely used pyrogen tests.
Light scattering offers distinct advantages for scientists interested in immunology. Find out how the NEPHELOstar Plus is used for high-throughput immunological tests.