Quality control and assurance validate production processes
For the maintenance of ordered production systems, it is essential to ensure the consistent and accurate quality of the production processes and consequently the final product. This applies in particular to products which are intended for human use such as foods, drugs, and medicinal products, or products applied to life stock or plants, since contaminants or falsely administered concentrations can lead to health issues or disease. The quality of a product is made up of various quality parameters. It is important to ensure consistent purity, composition and quantity. Microplate readers offer various solutions for determining these quality parameters.
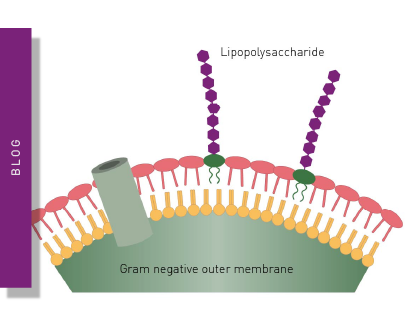
The determination of contaminants and impurities in a sample is an important prerequisite for many downstream applications of products. Such impurities can range from undesired side products that can arise in a production process to microbial contaminations and toxins that can accumulate in a product. Ordered production systems often also include purification procedures. However, these purification processes themselves also need to be validated to ensure product purity, since such procedures can be inefficient or even introduce additional or new contaminants. Microplate reader-based methods, for example for the analysis of DNA purity or the detection of endotoxins, are the ideal tools to accomplish this task since they combine sensitivity and minimal sample volumes with the ability to achieve high sample throughput. Microplate readers are used frequently to identify mycoplasma contaminations in cell culture with assay kits like MycoAlert®.
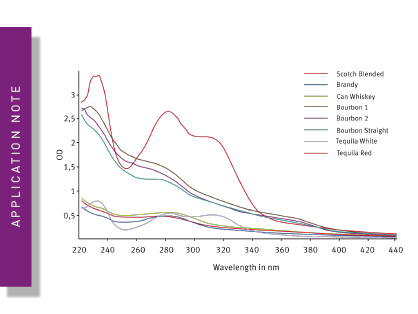
But product purity alone isn´t the only measure for performing adequate quality control. Even if a product is pure and free of contaminants it should have a consistent composition and stability. The application note Antibody Aggregation on a Plate Reader, presents an example to test the stability of manufactured antibodies.. Drugs and biologicals, in particular have to be administered precisely to induce their effect to a targeted extent, since over-dosage can quickly lead to undesirable side effects. This principle also applies to the field of life sciences, as experimental results can only be interpreted correctly if researchers know the accurate composition and concentrations of their samples. Many methods, such as the quantification of antioxidant capacity, protein quantification, reporter assays or ELISAs can be performed on microplate readers, offering cost-effective and accurate solutions for quality control testing.