
PHERAstar FSX
Powerful and most sensitive HTS plate reader
Although the concept of fluorescence gain has always been hard to grasp, it is a make-or-break parameter in microplate reading. In fact, incorrectly selected gain settings can lead either to saturation or to bad quality data.
 Dr Tobias Pusterla
Dr Tobias Pusterla
Fluorescence gain is probably the most mysterious measurement parameter microplate reader users encounter. If you are an audiophile or a musician, you are probably quite familiar with the concept of gain. For microplate reader users though, while parameters like excitation and emission wavelengths or the number of flashes are self-explanatory, the gain has always been hard to grasp. Despite this fact, it is a make-or-break setting, as an incorrectly selected fluorescence gain can make your data unusable.
In microplate readers, fluorescence intensity (FI) is detected by photomultiplier tubes (PMTs). A PMT multiplies the incoming light signal of a sample using the photoelectric effect and converts light into an electric signal. This is then quantified by fluorescence plate readers in Relative Fluorescence Units (RFU).
As there are neither standardised PMTs nor a standardised RFU scale, absolute counts cannot be compared between readers of different manufacturers. Accordingly, unlike absorbance, FI is not an absolute measurement. Moreover, the intensity of a fluorescent signal is usually relative to other measurements, to a refence measurement, or to the gain settings.
Fluorescence gain is defined as the voltage of the PMT at which the incoming fluorescent signal is amplified. Hence, it can be seen as an amplification factor that allows providing better data resolution.
The dynamic range with which samples are detected is defined as the ratio between the brightest and dimmest intensity that a microplate reader can quantify, and significantly affects data output and quality. The main parameter influencing the dynamic range is the fluorescence gain.
In fact, modifying the gain moves the detection dynamic range along the concentration curve of the assay or analyte. Importantly, the fluorescence gain does not modify the width of the dynamic range, only its position.
Typically, high gain values provide large amplification and are hence suitable for dim signals. Very bright signals instead need a lower gain as less signal amplification is required (fig. 1).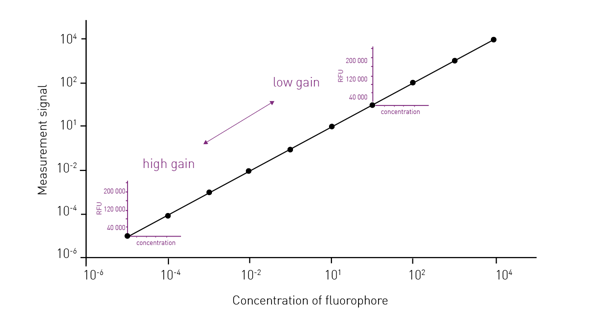
Inappropriate fluorescence gain values negatively affect data quality, assay window, and sensitivity. If bright samples are measured with a high fluorescence gain, this may result in the saturation of the detector and unusable data. On the contrary, if dim signals are detected with a low fluorescence gain, they may become indistinguishable from the background noise.
For more data examples highlighting the importance of appropriate gain values for your measurements, check out the HowTo Note: How to optimise the gain setting of your microplate reader?
To avoid these issues and provide the best possible dynamic range between the highest and the lowest measurement values of your assay, fluorescence gain is typically adjusted on the sample with the expected highest signal output (e.g., a positive control).
On microplate readers, the gain can be either determined empirically or can be automatically adjusted. The empirical determination relies either on trial and error or on pre-defined fluorescence gain values defined by microplate reader manufacturers for specific commercial assays.
The former method provides tailor-made parameters for a specific assay but implies time and cost expenditure, as depending on the nature of the assay or kit, measurements have to be repeated multiple times to identify a fluorescence gain value that avoids saturation but still covers a good assay dynamic range. The latter method, provides a safer approach but is usually not flexible and, depending on the assays to be measured, quite limiting.
All BMG LABTECH microplate readers can automatically determine the fluorescence gain, provided a specific well with a specific signal intensity is identified. Automatic gain adjustment involves a pre-measurement scan of your samples.
We typically recommend running an automatic gain adjustment at 90% capacity on the sample where the strongest signal is expected. The remaining 10% will give you a bit of buffer in case you have replicates with slightly varying signal intensity. If the sample with the strongest signal is not known and/or a positive control is not available, users can run an automatic gain adjustment at 10% capacity on a blank or negative control. However, this approach cannot avoid saturation, depending on the samples.
On the PHERAstar FSX, CLARIOstar Plus and the VANTAstar, fluorescence gain adjustments are no longer necessary. The Enhanced Dynamic Range (EDR) technology was specifically designed to offer the largest possible dynamic range - 8 concentration decades, making gain adjustments superfluous. With EDR, highly reliable results can be measured over a large dynamic range with no manual intervention.
EDR is extremely beneficial when running kinetic assays. In such assays signal intensity typically builds up over time, making an adjustment of the fluorescence gain upon detection complicated (fig. 3). A practical application of this principle is shown in the application note “CLARIOstar Plus simplifies enzymatic reaction monitoring with its novel Enhanced Dynamic Range (EDR) technology”
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Endpoint and kinetic mode assays are used by scientists to study many processes in the life sciences. Learn about endpoint and kinetic modes on a microplate reader.
The Z prime value (Z’) is a statistical parameter that can provide practicable information on the quality of an assay. This blog looks at its usage and describes some examples of specific applications.
Optical density and absorbance measurements are widely used in the life sciences. This blog looks at practical applications and some of the fundamentals.
Researchers have different technology options available for absorbance measurements. This blog compares spectrometers and monochromators. What’s the difference?
ELISAs are a popular tool to detect or measure biological molecules in the life sciences. Find out how microplate readers can be used to advance research using immunoassays.
LanthaScreen is a TR-FRET technology which can be used to measure kinase activity, compound binding, and post-translation modification events. Read more about this type of assay here.