Introduction
With the development of more sensitive technologies, further miniaturization of cell-based assays for high-throughput screening is now very feasible. Performing cell-based assays in sub microliter volumes, translates into very low compound requirement and fewer cells used. In addition to being more economical, more experiments can be done in the same amount of time and at the same overall price.
Combining technologies from three companies, an nHTS platform was developed to screen GPCRs (shown here) and kinases (data not shown here) in a cell-based assay format in 1536 and 3456-well plates. Technologies used include DiscoverX’s PathHunter® GPCR beta- Arrestin and PathHunter® Cell-based Kinase assay platforms; Labcyte’s Echo® liquid handling system to transfer cells and compounds; and BMG LABTECH’s PHERAstar FS microplate reader for low volume detection.
Assay Principle
The PathHunter® technology from DiscoverX is an adaptation of EFC that provides a novel cell-based assay format for detecting protein-protein interaction. In this approach, ProLink™ is appended to GPCR (1B) or RTK or cytokine receptor (1C), while EA is recombinantly expressed as a fusion protein with β-Arrestin (Fig 1B) or SH2 phosphotyrosine domain (Fig. 1C). Activation of the receptor upon ligand binding allows interaction of GPCR-β-Arrestin or RTK-SH2 protein resulting in a measurable chemiluminescent signal.
Materials & Methods
- PHERAstar FS from BMG LABTECH
- Echo® 555 liquid handler from Labcyte PathHunter® cell lines and assay reagents from DiscovrX
- 1536- and 3456-well microplates from Aurora
Cell Handling
PathHunter® Cells were diluted in Hank’s Buffered SalineSolution. 40 μL were added to Echo® qualified 384-well polypropylene microplates (P-05525). PathHunter® Cells were transferred to an Aurora 1536-well or 3456-well microplate using an Echo® 555 liquid handler with a 384PP_AQ_SP calibration. Cell volumes were varied as part of the testing plan. Compounds such as pergolide, ProLactin or β-NGF were diluted in DMSO and added to an Echo® qualified 384-well polypropylene microplate and transferred at 30 nL using the 384PP_DMSO calibration. Incubation as per β-arrestin and Cell-based Kinase Assay recommendations.
Addition of Detection Reagents
PathHunter® Flash Detection reagent was transferred from an Echo® qualified 384-well microplate and transferred (at a 1:1 ratio of cell volume: PathHunter® Flash Detection reagent) with the Echo® 555 liquid handler with the 384PP_AQ_SP calibration. Incubate for 30 mns at RT.
Detection
Both 1536- and 3456-well microplates were measured on the PHERAstar FS using a measurement time of 0.1 sec/well at a gain of 3600 with a luminescence optic module. An automatic z-height adjustment was performed on the well with the highest signal before reading the plate. The 0.1 mm adjustable z-height allows the PHERAstar FS to obtain the highest possible signal-to-noise ratios in all cell-based assays.
Results & Discussion
Adherent Format
PathHunter® U2OS cells expressing Trk A and PRLR-JAK1 at 1000 cells/well were seeded in a white TC coated AURORA 1536 plates and stimulated with known agonists β-NGF and prolactin. These plates were incubated for 3 hrs at 22°C. A robust assay response is measured with 6 μL total volume (Figure 2).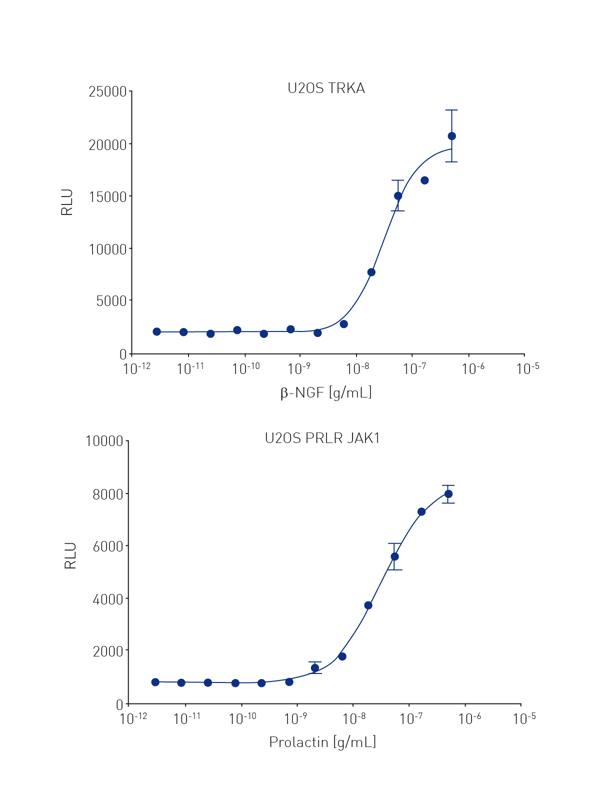
These results indicate that the PathHunter® arrestin (and kinase) assay can be successfully adapted to detection in 1536 well microtiter plates using adherent cells.
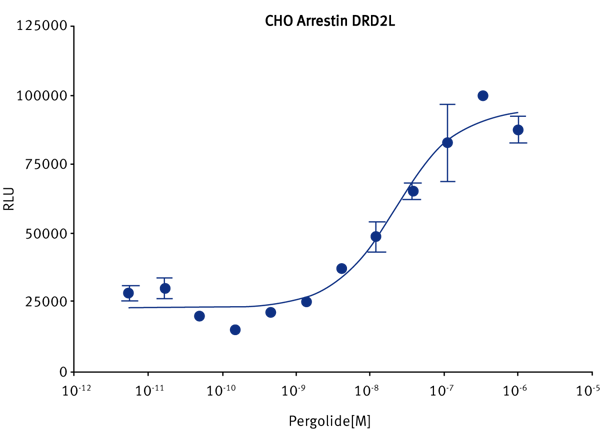
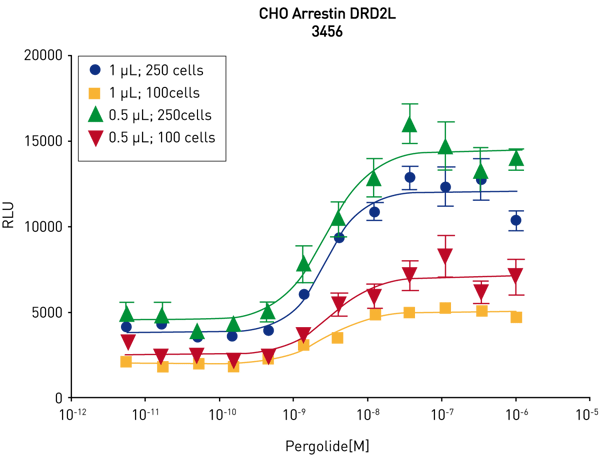 The protocol for cell seeding using the Echo® 555 was applied to cell dispensing into 3456 well plates, figure 5. The performance of different cell densities was tested to determine the optimal cell number in the new plate format. PathHunter® DRD2L cells 100 or 250 cells/well (in suspension) were added to the AURORA 3456 well plates in 0.5 or 1 μL volumes via the Echo® instrument. Compound (5nl of 100X in DMSO) was added to the cells plated in 0.5 μl (10 nl for the cells plated in 1μl) via the Echo® instrument.
The protocol for cell seeding using the Echo® 555 was applied to cell dispensing into 3456 well plates, figure 5. The performance of different cell densities was tested to determine the optimal cell number in the new plate format. PathHunter® DRD2L cells 100 or 250 cells/well (in suspension) were added to the AURORA 3456 well plates in 0.5 or 1 μL volumes via the Echo® instrument. Compound (5nl of 100X in DMSO) was added to the cells plated in 0.5 μl (10 nl for the cells plated in 1μl) via the Echo® instrument.
Conclusion
Miniaturization with highly evolved automation technologies affords the benefit of faster screens at much lower costs. Here we have demonstrated the application of the DiscoverX’s PathHunter GPCR and cell-based kinase assays in 3456 and 1536 platforms using Labcyte’s ECHO Liquid handler and BMG LABTECH’s PHERAstar FS. We describe an nHTS cell-based screening platform that can measure as little at 250 cells per well using less than 1 microliter.