Introduction
The Transcreener® assays from BellBrook Labs offer a flexible approach to detect enzyme activity in high throughput screening (HTS) format. The assay is based on the direct detection of nucleotides such as ADP, GDP, UDP, GMP and AMP allowing determining the activity of e.g. methyltransferases, acetyltransferases, kinases or GTPases. The assays use far-red dyes and are available in three detection modes:
- Transcreener FP (Fluorescence Polarization)
- Transcreener FI (Fluorescence Intensity)
- Transcreener TR-FRET (Time-Resolved Fluorescence Resonance Energy Transfer)
In this application note we show the performance of the PHERAstar FSX microplate reader from BMG LABTECH in Transcreener® assays. In validation measurements the output values resulted in high sensitivity data while the read time per 384-well microplate can be < 30 sec. confirming the instrument to be an excellent option for HTS.
Assay Principle
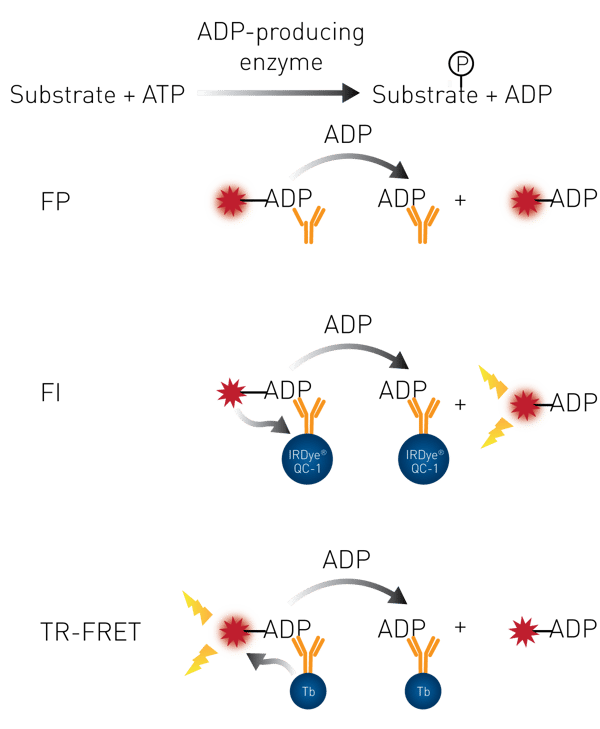
After the enzymatic reaction ADP is present in the sample. A general description of the different kinds of ADP detection is presented in Fig. 1.
Transcreener FP principle
ADP-labeled with Alexa633 and conjugated to an antibody is added to the well. ADP that is produced during the enzymatic reaction will compete with the Alexa633-ADP for the binding site of the ADP antibody and will displace the Alexa633-ADP. This will result in a de-creased FP value.
Transcreener FI principle
ADP-labeled with Alexa594 is conjugated to an antibody that carries a quencher. ADP that is produced during the enzymatic reaction will compete with the Alexa594-ADP for the binding site of the ADP antibody and will displace the Alexa594-ADP. That brings fluorescent dye and quencher into distance resulting in an increase in FI value.
Transcreener TR-FRET principle
ADP-labeled with acceptor dye HiLyte647 is conjugated to an antibody that carries donor terbium chelate. ADP that is produced during the enzymatic reaction will compete with the HiLyte647-ADP for the binding site of the ADP antibody and will displace the labeled ADP. That brings TR-FRET donor and acceptor out of proximity and leads to a decrease in TR-FRET signal.
Materials & Methods
- Transcreener® ADP2 FP, FI, TR-FRET assay kits
- Black 384-well small volume, low binding plate (Greiner)
- PHERAstar FSX microplate reader
ADP/ATP standards were prepared to mimic an enzymatic reaction. 10 μM ADP and 10 μM ATP stock solutions were combined at varied proportions to create different percent conversions of ATP to ADP ranging from 0 to 10 μM. 10 μl of standard and 10 μl of ADP detection mixture (containing ADP antibody and ADP far red tracers) are combined in the microplate and incubated for 1 hour at room temperature. After incubation the plate was measured in the PHERAstar FSX.
PHERAstar FSX instrument settings
| Transcreener FP | Transcreener FI | Transcreener TR-FRET | |||
| Simultaneous Dual Emission | yes | yes | |||
| Optic module | FP 590 675 675 | FI 580 620 | TRF 337 665 620 | ||
| Excitation source | Flash lamp | Flash lamp | Laser or flash lamp | ||
| Int. start: 50 μs Int. time: 50 μs |
|||||
|
Gain/Focus |
Should be adjusted prior the measurement |
||||
Results & Discussion
Transcreener FP
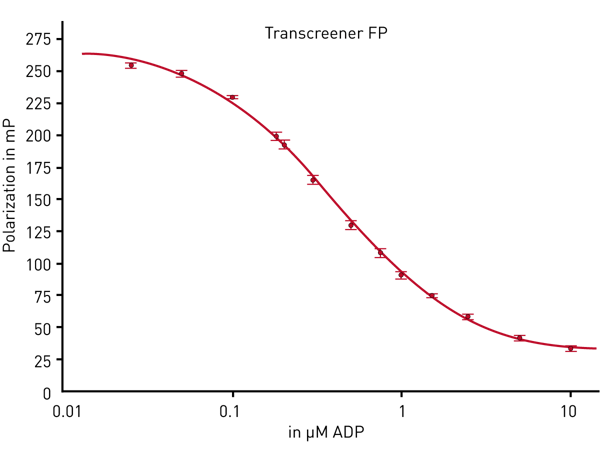
Fig. 2 shows that with the PHERAstar FSX an assay window higher than 200 mP has been reached (assay window = values for 100 % ATP- values for 100 % ADP).
The effect of the number of flashes on robustness of measurement is shown in Table 1.
| No. of f ashes | 100 | 50 | 25 | 13 | ||
| Δ mP at 10 % conversion | 156 | 157 | 158 | 158 | ||
| Z‘ at 10 % conversion | 0.878 | 0.819 | 0.780 | 0.749 | ||
| Read time for a full 384w plate | 3 min 31 sec |
2 min 26 sec |
1 min 53 sec |
1 min 37 sec |
||
The assay can be reliably measured in less than 2 min for a whole 384-well microplate.
Transcreener FI
Transcreener FI signals increase with increasing ADP concentrations. According to the certification requirements it is necessary to obtain a Z´ factor of 0.7 at 10 % ATP conversion. Fig. 3 shows that with the PHERAstar FSX this Z´factor is achieved at 4% conversion, much lower than the certification requirements.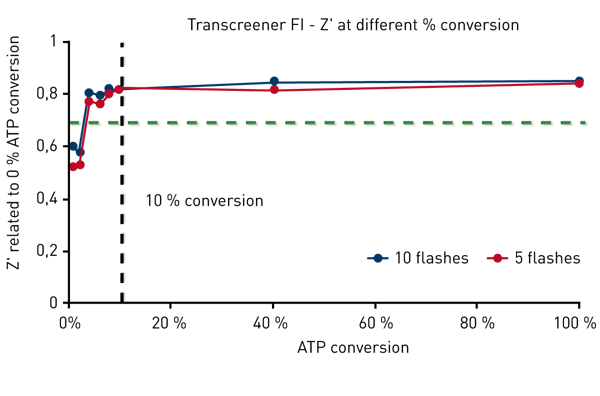 Transcreener TR-FRET
Transcreener TR-FRET
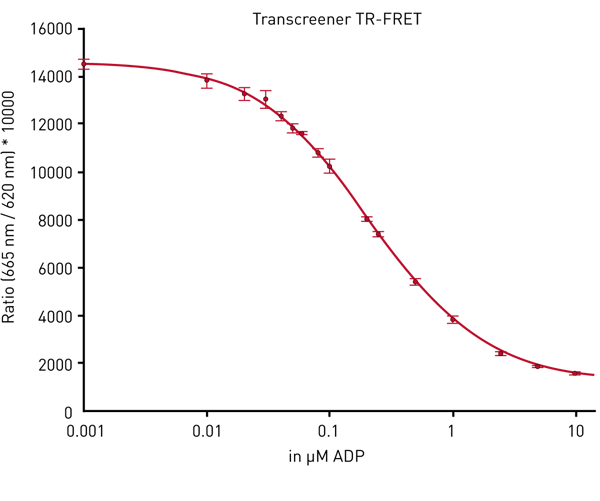
TR-FRET measurements can be performed by either using the flash lamp or the laser in the PHERAstar FSX. A standard curve obtained with the laser is shown in Fig. 4.
Flash lamp and laser give comparable data in terms of assay window and stability of data. The advantage of the laser is the use of a less number of flashes. In the flying mode the whole plate can be read in less than 30 sec (Table 2).
| Flashes Flash lamp | Z‘ value | Read time | Flashes Laser | Z‘ value |
Read time |
||
| 50 | 0.815 | 2 min 27 sec |
10 | 0.896 | 2 min 21 sec |
||
| 25 | 0.807 | 1 min 53 sec |
5 | 0.884 | 1 min 49 sec |
||
| 10 | 0.747 | 1 min 33 sec |
1 | 0.830 | 1 min 23 sec |
||
| 5 | 0.746 |
1 min 26 sec |
flying | 0.736 | 27 sec | ||
Validation criteria from BellBrook Labs
- 384-well format
- Z´- Factor ≥ 0.7 at 10 % conversion of 10 μM ATP
- Δ mP ≥ 95 mP at 10 % conversion of 10 μM ATP
- Read Times to achieve specifications ≤ 5 minutes
Conclusion
Based on the data shown the PHERAstar FSX has obtained all three Transcreener certifications.