Introduction
The potential for cardiotoxic side-effects continues to challenge the development of small molecule based therapies. These intolerable side-effects are often precipitated by drug-induced long QT syndrome (LQT), which is often linked to blocking the human ether-a-go-go related gene (hERG) potassium channel. Although patch-clamp electrophysiology remains the gold standard for determining the interaction of compounds with the function of the hERG channel, radioligand displacement assays have proven to be a cost-effective initial alternative assay for hERG channel liability at early stages of compound development. Speed and cost still suffer however, as radioligand displacement assays remain heterogeneous and depend on the procurement and disposal costs of radioligands. Because the FDA has recommended that all new drug candidates be tested for blockage of the hERG channel, there is a pressing need for a simple, safe, cost-effective method to triage compounds earlier in the discovery process.
To address this need, the PredictorTM hERG Fluorescence Polarization Assay Kit from Invitrogen has been developed. The assay is based upon the principle of fluorescence polarization (FP), in which a red-shifted fluorescent tracer is displaced from the hERG channel by compounds that bind to the channel. When the tracer is bound to the channel the FP value is high, and when displaced by hERG binding compounds the FP value is low (Figure 1).
The assay kit includes a stable membrane preparation that contains high levels of hERG protein, as well as optimized tracer, buffer, and controls necessary to perform the assay. This technical note describes the performance of the PredictorTM hERG Assay on the BMG LABTECH’s PHERAstar® FS multifunctional HTS microplate reader.
BMG LABTECH’s PHERAstar FS is a multidetection microplate reader that combines rapid plate reading necessary for high throughput screening (HTS) with enhanced performance and sensitivity needed to read small fluid volumes. The PHERAstar FS has been designed to read all HTS detection modes (fluorescence intensity, time-resolved fluorescence, fluorescence polarization, luminescence, and absorption) in all plate formats up to 1536 wells. The PHERAstar FS uses a unique application-specific optic module in conjunction with a reading head featuring five photomultiplier tubes that can simultaneously measure two emission signals at any desired wavelength. This Simultaneous Dual Emission (SDE) detection on the PHERAstar FS provides for outstanding sensitivity and accuracy in fluorescence polarization assays, minimizing read times and increasing Z’ values.
Materials & Methods
- PHERAstar FS microplate reader with PredictorTM FP module (540-20/590-20), BMG LABTECH
- Black 384 well low volume microplates from Corning
- PredictorTM hERG Fluorescence Polarization Assay Kit from Invitrogen
Follow the PredictorTM hERG Fluorescence Polarization Assay Kit instructions. Briefly, a 10 μL dilution series of the compound was placed in a 384 well plate (in duplicate), followed by 5 μL additions of both tracer and hERG membrane preparations. The plate could then be read any time between a 1 and 24 hour (or more) incubation.
Results & Discussion
Competitive Binding of Known hERG Blockers
Ten compounds that have been previously shown to block the hERG channel were tested in the PredictorTM hERG Fluorescence Polarization Assay using the BMG LABTECH PHERAstar FS (Fig. 2). 
The polarization data are shown in Figure 2, with the data summarized and compared with patch-clamp or radioligand displacement data that have been previously reported in Table 1. Non-specific displacement of the tracer from the membrane in the FP assay (observed with some compounds at high concentrations) was corrected by fixing the baseline of the displacement curve to the value seen with saturating levels (30 μM) of E-4031, a compound for which no non-specific displacement of tracer was observed.
| IC50 (nM) |
|||
| Compound | PredictorTM FP | Patch Clamp | Radioligand |
| E-4031 | 27 | 48 | 20 |
| amitriptyline |
7118 | 10000 | 2440 |
| haloperidol | 167 | 174 | 90 |
| fluoxetine | 2687 | 990 | 2230 |
| dofetilide | 11 | 12 | 40 |
| astemizole | 3 | 1.2 | 1 |
| terfenadine | 23 | 16 | 30 |
| pimozide | 8 | 18 | 6 |
| thioridazine | 553 | 1250 | 510 |
| bepridil | 230 | 550 | 170 |
Determination of Assay Z’ Value
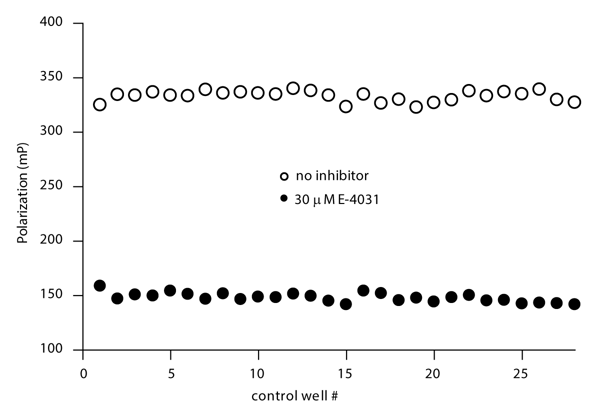
Assay robustness was assessed by measuring control wells containing either 30 μM E-4031 (to completely displace tracer from binding to hERG) or no compound. All wells contained 1 % DMSO. The data are shown in Figure 3 and a Z’ value of 0.86 was determined. Although not shown here, the assay is stable at DMSO, methanol, or ethanol concentrations up to 5 %.
Conclusion
Because of the potentially lethal effects of unintended hERG block, the FDA has recommended that all compounds be functionally tested for this effect in an electrophysiological (patch-clamp) assay before being tested in humans. Due to the need for highly specialized equipment and expertise, this test is typically performed only at the later stages of the discovery process. While assessment of hERG channel function will remain the gold standard to assess hERG channel block, binding assays can be used in order to triage compounds and identify potential liabilities earlier in the process. The recently developed PredictorTM hERG Fluorescence Polarization Assay Kit provides a homogenous assay format that avoids the problems of using radioisotopes to meet this need. In addition, the PHERAstar FS multifunctional HTS microplate reader provides one of the fastest and most sensitive platforms to run this FP assay.